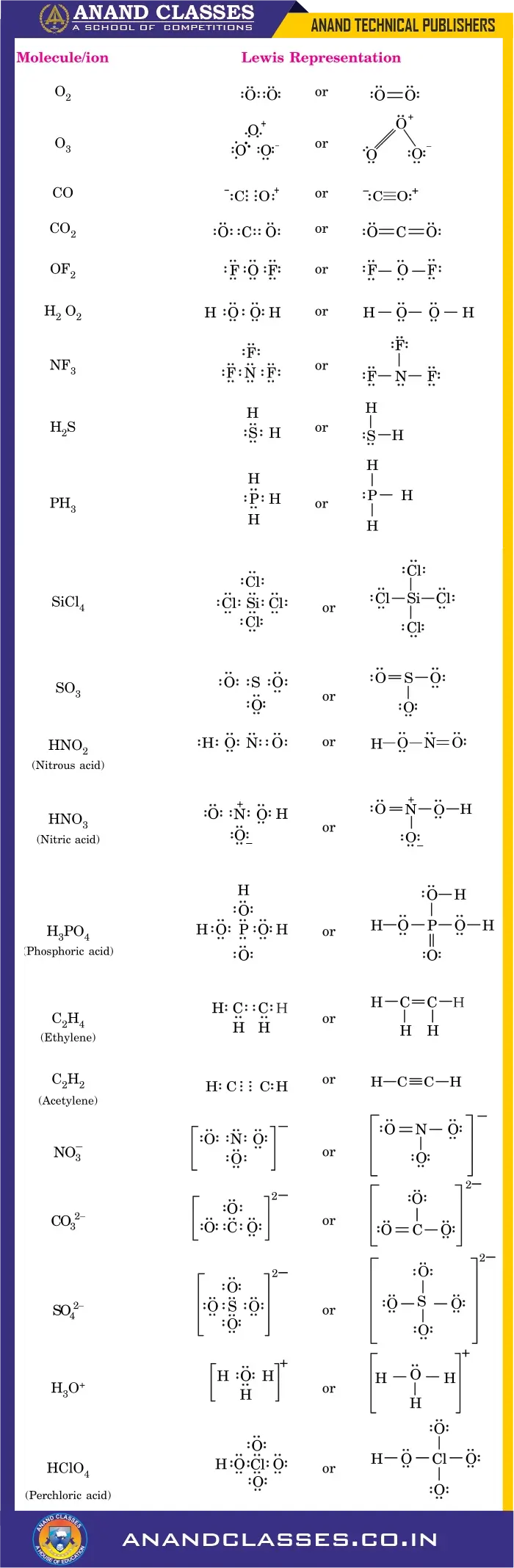
Anand Classes offers comprehensive notes on the Important Lewis Structures of Covalent Compounds and Ions for Class 11 Chemistry. These structures include step-by-step representation of molecules such as $CH_4$, $CO_2$, $NH_3$, $H_2O$, $BF_3$, as well as polyatomic ions like $NO_2^-$, $CO_3^{2-}$, and $SO_4^{2-}$. Each structure is explained using the octet rule, resonance concept, and bond formation, making it easier for students to prepare for CBSE, JEE, and NEET examinations. Click the print button to download study material and notes.
Lewis Dot Structures of Important Molecules or Ions
FAQs on Important Lewis Structures
Q1. What are Lewis structures?
Lewis structures (or electron dot structures) represent how valence electrons are arranged in covalent compounds and ions using dots (for electrons) and lines (for bonds).
Q2. Why are Lewis structures important?
They help predict molecular shape, bond order, resonance, and stability of molecules/ions.
Q3. Which molecules and ions are most important for Class 11 exams?
Common examples: $CH_4$, $NH_3$, $H_2O$, $CO_2$, $BF_3$, $O_2$, $N_2$, $NO_2^-$, $CO_3^{2-}$, $SO_4^{2-}$.
Q4. What is resonance in Lewis structures?
Resonance occurs when more than one valid Lewis structure can be drawn for a molecule/ion (e.g., $NO_2^-$, $CO_3^{2-}$).
Q5. Do all compounds follow the octet rule?
No, exceptions exist. For example, $BF_3$ is electron-deficient and $PCl_5$ has an expanded octet.
MCQs on Lewis Structures
Q1. The Lewis structure of $CH_4$ contains:
(a) 4 single bonds
(b) 2 double bonds
(c) 1 double and 2 single bonds
(d) 1 triple bond
Answer: (a) 4 single bonds
Q2. Total number of valence electrons in $CO_3^{2-}$ is:
(a) 22
(b) 24
(c) 26
(d) 28
Answer: (b) 24
Q3. Which of the following species shows resonance?
(a) $CH_4$
(b) $CO_2$
(c) $CO_3^{2-}$
(d) $NH_3$
Answer: (c) $CO_3^{2-}$
Q4. In the Lewis structure of $NH_3$, nitrogen has:
(a) 3 bond pairs, 1 lone pair
(b) 4 bond pairs
(c) 2 bond pairs, 2 lone pairs
(d) 1 bond pair, 3 lone pairs
Answer: (a) 3 bond pairs, 1 lone pair
Assertion–Reason Questions on Lewis Structures
Q1.
Assertion (A): $CO_2$ has a linear structure.
Reason (R): Carbon forms two double bonds with oxygen atoms, satisfying the octet rule.
- (a) A and R are true, R is the correct explanation of A
- (b) A and R are true, R is not the correct explanation of A
- (c) A is true, R is false
- (d) A is false, R is true
Answer: (a)
Q2.
Assertion (A): $BF_3$ follows the octet rule.
Reason (R): Boron has 8 valence electrons in $BF_3$.
- (a) A and R are true, R is the correct explanation of A
- (b) A and R are true, R is not the correct explanation of A
- (c) A is true, R is false
- (d) A is false, R is true
Answer: (d)
Case Study Question based on Lewis Structures
Case:
A student is asked to draw the Lewis structure of $CO_3^{2-}$. He calculates total valence electrons:
- Carbon = 4
- Oxygen = $3 \times 6 = 18$
- Extra electrons for charge = 2
- Total = 24
He connects carbon with three oxygens and tries to complete octets.
Questions:
- Why is resonance required in $CO_3^{2-}$?
- How many bond pairs and lone pairs are present in one resonating structure?
Answers:
- Resonance is required because a single Lewis structure cannot represent delocalized $\pi$ electrons.
- Bond pairs = 4 (one double + two single bonds), Lone pairs = 8 (two on each O).


