Anand Classes provides comprehensive Class 11 Chemistry notes on Dipole Moment and Polarity of Molecules with clear definitions, important concepts, solved Q&A, MCQs, assertion-reason questions, and case study-based problems. Learn how the dipole moment arises from differences in electronegativity and molecular geometry, and understand how it determines whether a molecule is polar or non-polar. These exam-focused notes explain key examples like H2O, NH3, CO2, BF3, and CHCl4 making it easy to revise and score high in NEET, JEE, and CBSE board exams. Click the print button to download study material and notes.
What is Electric Dipole in a Molecule ?
In a polar molecule, the centre of negative charge does not coincide with the centre of positive charge.
Such molecules, having two equal and opposite charges separated by a certain distance, are said to possess an electric dipole.
Example: HCl molecule behaves like dipole.
Although the molecule is electrically neutral, the negative charge is equal in magnitude to the positive charge.
What is Dipole Moment ?
The dipole moment is defined as:
The product of the magnitude of either charge and the distance of separation between the two charges.
It is represented by the Greek μ.
Mathematically:
μ = q x d
where,
- q = charge on any of the atoms
- d = distance between the atoms
What is CGS Units of Dipole Moment ?
- The charge q is of the order of 10-10 esu
- The bond length d is of the order of 10-8 cm (1 Å)
Hence,
μ = 10-10 x 10-8 = 10-18 esu·cm
This unit is called 1 Debye (D).
Thus,
1 D = = 10-18 esu·cm
Examples:
- Dipole moment of HCl = 1.03 D
- Dipole moment of H2O = 1.85 D
How to convert 1 Debye to SI Units of Dipole Moment ?
In SI system, dipole moment is expressed in C·m.
We know,
$$1 \; \text{esu} = \frac{1.602 \times 10^{-19} \; C}{4.803 \times 10^{-10}} = 3.335 \times 10^{-10} \; C$$
and
$$1 \; \text{cm} = 10^{-2} \; m$$
Therefore,
$$1 \; D = 10^{-18} \; \text{esu·cm}$$
$$1 \; D = 10^{-18} \times (3.335 \times 10^{-10} \; C) \times (10^{-2} \; m)$$
$$1 \; D = 3.335 \times 10^{-30} \; C·m$$
Vector Nature of Dipole Moment
Dipole moment is a vector quantity.
It is represented by an arrow pointing from positive to negative charge.
Representation:
$$H^{\delta^+} \longrightarrow Cl^{\delta^-}$$
The crossed arrow ((+ end) ${\longrightarrow}$ (-end)) is also used above the Lewis structure to indicate the direction of electron density shift.
What is Dipole Moment and Molecular Structure?
There is a relation between dipole moment and molecular structure because dipole moment is a vector quantity and from information of molecular structure, compute the dipole moment of a molecule.
What is the dipole moment of Diatomic Molecules?
A diatomic molecule has two atoms bonded by a covalent bond.
- In such a molecule, the dipole moment of the bond (bond dipole) is the same as the dipole moment of the molecule.
- A diatomic molecule is polar if the bond between atoms is polar.
Example:
Dipole moment of $HCl$ molecule is the same as that of the $H–Cl$ bond:
$$\mu = 1.07 , D$$
Greater the electronegativity difference between the atoms, more will be the dipole moment of such molecules.
👉 Greater the electronegativity difference → larger the dipole moment.
For example, the dipole moment of hydrogen halides decreases with decreasing electronegativity of the halogen atom.
Dipole Moment Trend in Hydrogen Halides :
| Molecule | Electronegativity of Halogen | Dipole Moment (D) |
|---|---|---|
| $H–F$ | 4.0 | 1.78 |
| $H–Cl$ | 3.0 | 1.07 |
| $H–Br$ | 2.8 | 0.79 |
| $H–I$ | 2.5 | 0.38 |
What is the dipole moment of Polyatomic Molecules?
In polyatomic molecules (more than two atoms), dipole moment depends not only on individual bond dipoles but also on the spatial arrangement of bonds.
In such cases, the dipole moment of a molecule is the vector sum of the dipole moments of various bonds.
Why is Dipole Moment of CO2 Zero but That of H2O Non-Zero yet both has polar bonds ?
Carbon dioxide (CO2) and water (H2O) are both triatomic molecules but dipole moment of carbon dioxide is zero whereas, that of water is 1.84 D. This can be explained on the basis of their structures.
Carbon dioxide is a linear molecule in which the two C = O bonds are oriented in the opposite directions at an angle of 180°.
The dipole moment of each C =O bond is 2.3 D but due to linear geometry of CO2, the dipole moment of one C = O bond cancels that of another. Therefore, the resultant dipole moment of the molecule is zero.
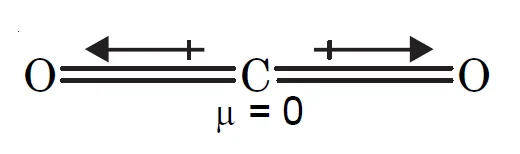
Hence, CO2 is a non-polar molecule yet it has polar bonds.
Water molecule has a bent structure in which the two O—H bonds are oriented at an angle of 104.5°. The dipole moment of water is 1.85 D (or 1.85 × 3.335 × 10–30 C m = 6.17 × 10–30 C m) , which is the resultant of the dipole moments of two O—H bonds. Therefore water is a polar molecule.
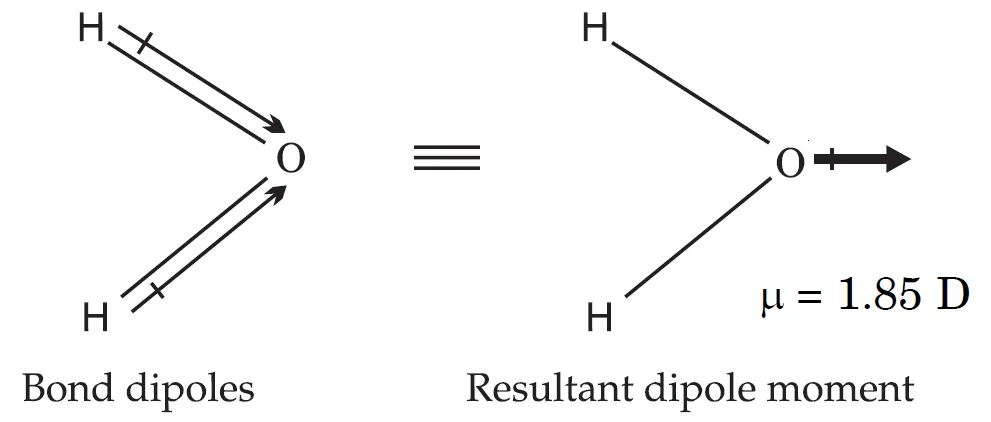
Summary
- CO2: Linear molecule (180o).
- Each C=O bond has μ = 2.3 D.
- Bond dipoles cancel.
- CO2 , μ = 0 D → non-polar.
- H2O: Bent molecule (104.5o).
- Two O–H dipoles add up.
- H2O = 1.85 , μ = 6.17 x 10-30 C·m → polar molecule.
Why Does BeF2 Have Zero Dipole Moment?
The dipole moment in BeF2 is zero. This is because the molecule is linear and the two equal bond dipoles point in opposite directions. These bond dipoles cancel the effect of each other giving a net zero dipole moment.
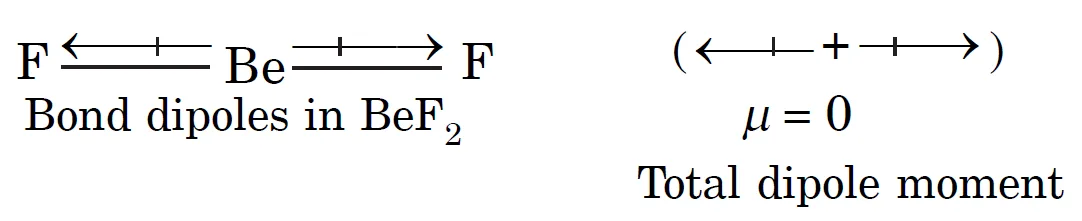
Summary
- BeF2 is linear.
- Two equal bond dipoles point in opposite directions.
- Dipoles cancel → μ of BeF2 = 0 D
Why is BF3 Non-Polar but NH3 Polar?
Tetra-atomic molecules such as BF3 and NH3. The dipole moment of BF3 molecule is zero while that of NH3 is 1.47 D.
This suggests that BF3 molecule has symmetrical structure in which the three B—F bonds are oriented at an angle of 120° to one another. The three bonds lie in one plane and the dipole moments of these bonds cancel one another giving net dipole moment equal to zero.
On the other hand, the ammonia molecule has pyramidal structure. The individual bond dipole moments of three N—H bonds give the resultant dipole moment of NH3 molecule as 1.47 D.
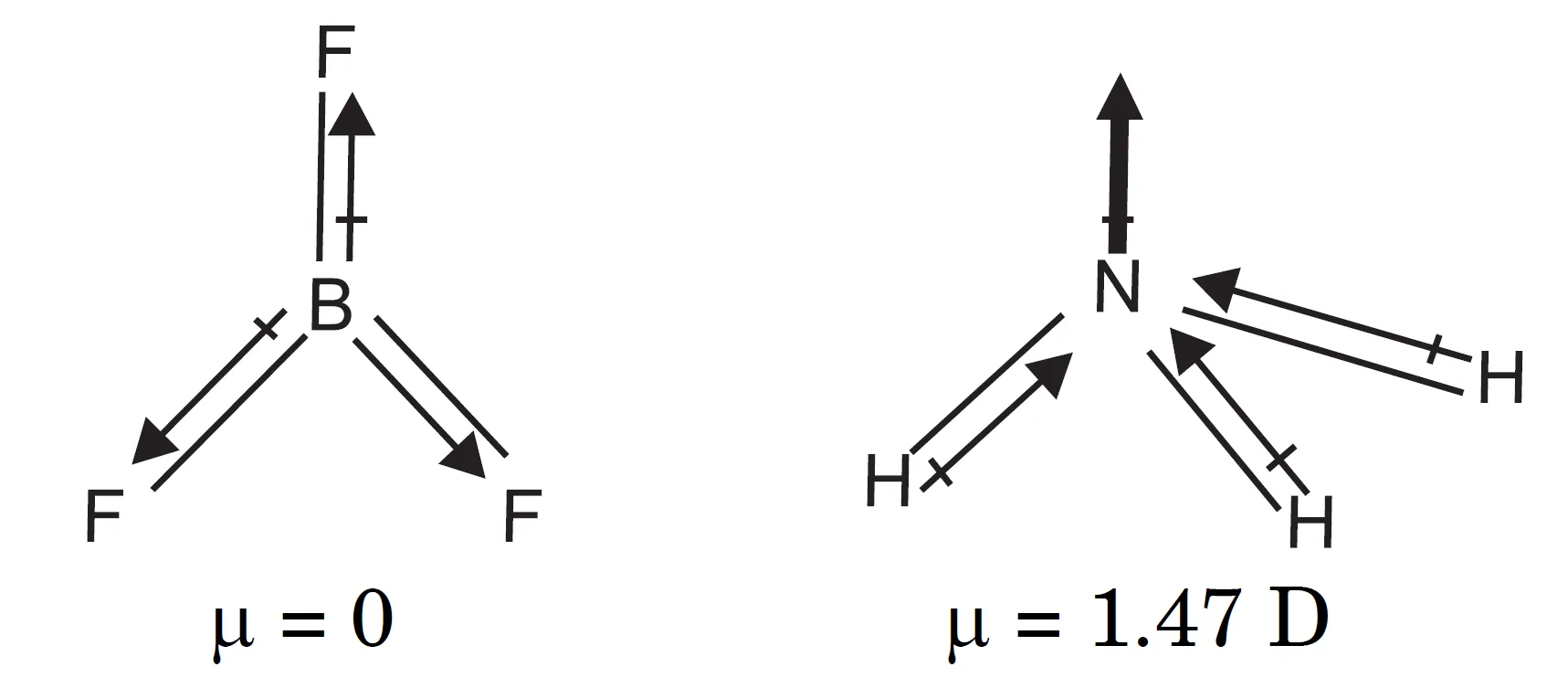
- BF3: Trigonal planar, 120o.
- Three B–F bond dipoles cancel.
- μ of BF3 = 0 D → non-polar.
- NH3: Trigonal pyramidal.
- Three N–H bond dipoles add to give net resultant.
- μ of NH3 = 1.47 D → polar molecule.
Note : Thus, it may be concluded that the presence of polar bonds in a polyatomic molecule does not mean that the molecule as a whole will always have dipole moment.
Why Do Some Symmetrical Tetrahedral Molecules Have Zero Dipole Moment?
Polar bonds in a polyatomic molecule does not mean that the molecule as a whole will always have dipole moment. In some cases, the arrangement of individual bonds in the molecule is such that the net dipole moment of the molecule is zero.
The net dipole moments of carbon dioxide and boron trifluoride molecules are zero.
Similarly, the dipole moments of methane and carbon tetrachloride molecules are zero because of the symmetrical tetrahedral shapes of these molecules. The dipole moments of these bonds cancel one another giving net dipole moment equal to zero.
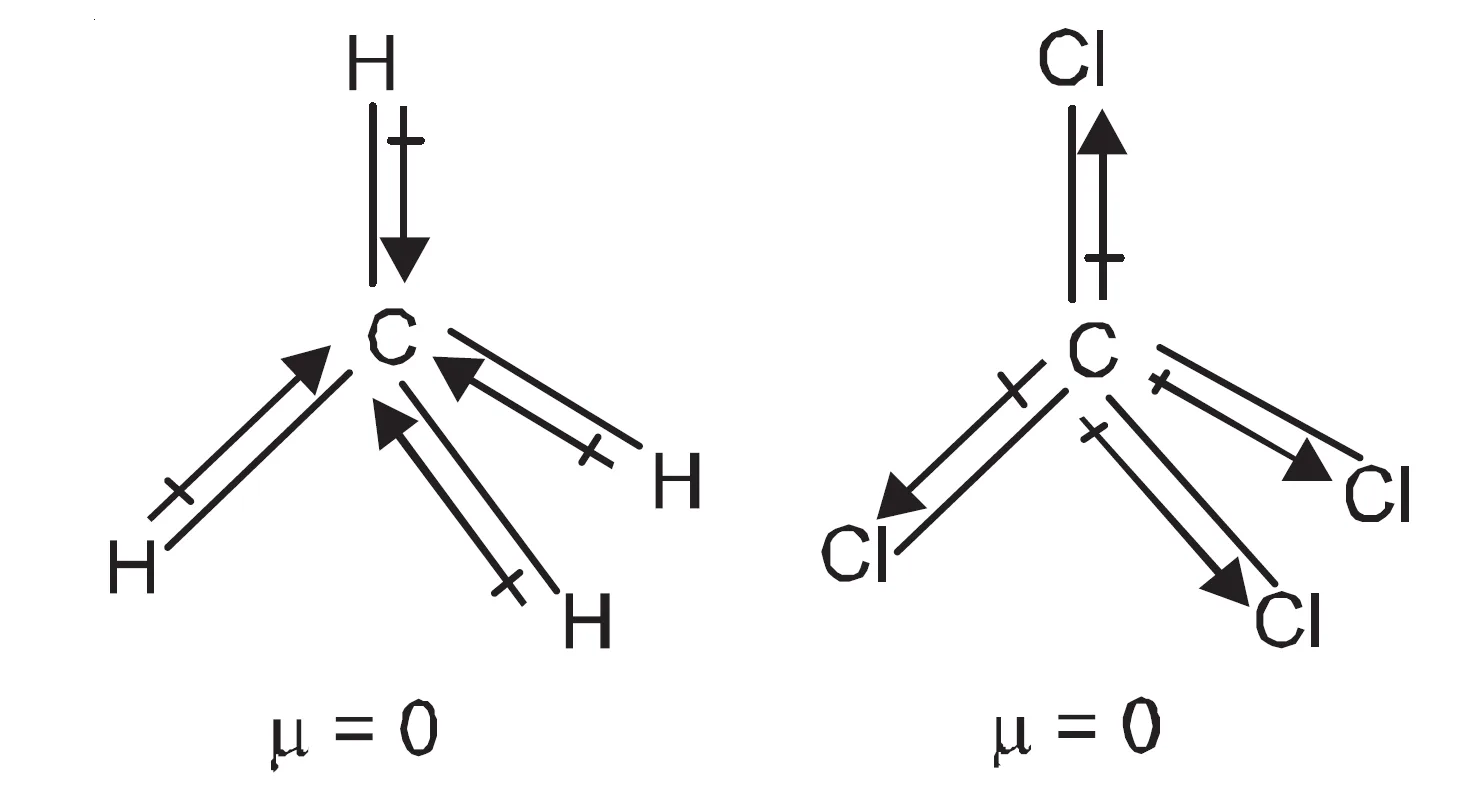
- CH4 and CCl4: Tetrahedral & symmetrical.
- Bond dipoles cancel.
- μ of CH4 and CCl4 = 0 D
Why is Dipole Moment of CHCl3 (chloroform) Non-Zero?
The dipole moment of CHCl3 (chloroform) is not zero because all the bonds are not same. The resultant of bond dipole moments of three C—Cl bonds is not cancelled by bond dipole moment of C—H bond.
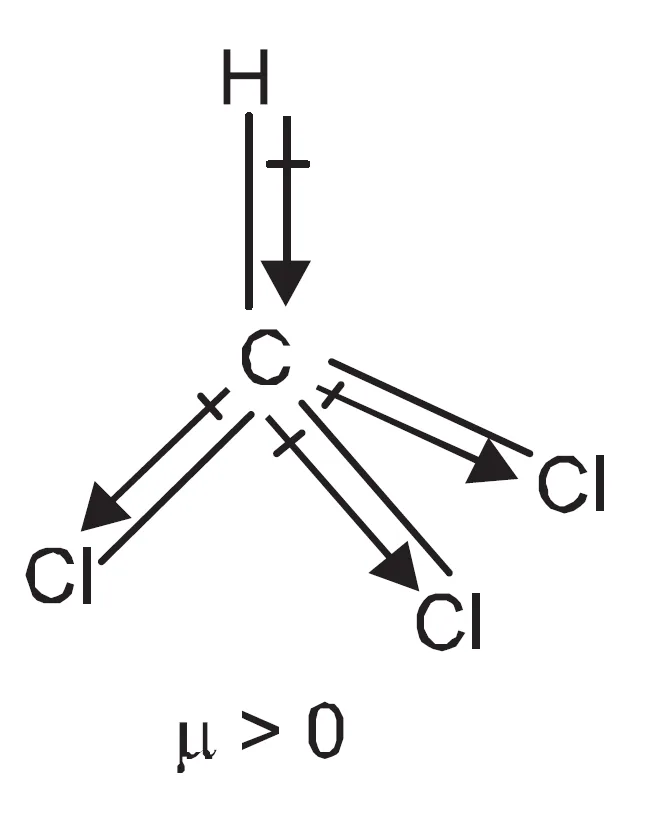
Summary
- CHCl3: Tetrahedral but bonds not identical.
- Three C–Cl bond dipoles are not fully cancelled by C–H bond dipole.
- Hence, CHCl3 has a non-zero dipole moment.
Why is the Dipole Moment of NH3 Greater than that of NF3 ?
NH3 and NF3 molecules present an interesting case in relation to net dipole moment.
- Both these molecules have pyramidal shape with one lone pair on the nitrogen atom.
- Fluorine is more electronegative than hydrogen, and therefore, the N–F bonds should be more polar than N–H bonds.
- Consequently, the resultant dipole moment of NF3 should be much larger than that of NH3.
However, the dipole moment of ammonia NH3 (μ = 1.47 D) is larger than that of NF3 (μ = 0.24 D).
This anomalous behaviour can be explained due to the presence of the lone pair on nitrogen:
- In case of NH3:
- Nitrogen is more electronegative than hydrogen [electronegativity of N = 3.0, H = 2.1].
- Therefore, the dipole of each N–H bond is from H → N.
- The orbital dipole due to the lone pair is in the same direction as the resultant dipole moment of the three N–H bonds.
- Hence, it adds up to the resultant dipole moment of the N–H bonds.
- In case of NF3:
- Fluorine is more electronegative than nitrogen [electronegativity of F = 4.0, N = 3.0].
- Therefore, the dipole of each N–F bond is from N → F.
- The orbital dipole of the lone pair is in the opposite direction to the resultant dipole moment of the three N–F bonds.
- Thus, the lone pair moment cancels the resultant N–F bond moments.
Consequently, the dipole moment of NF3 is low.
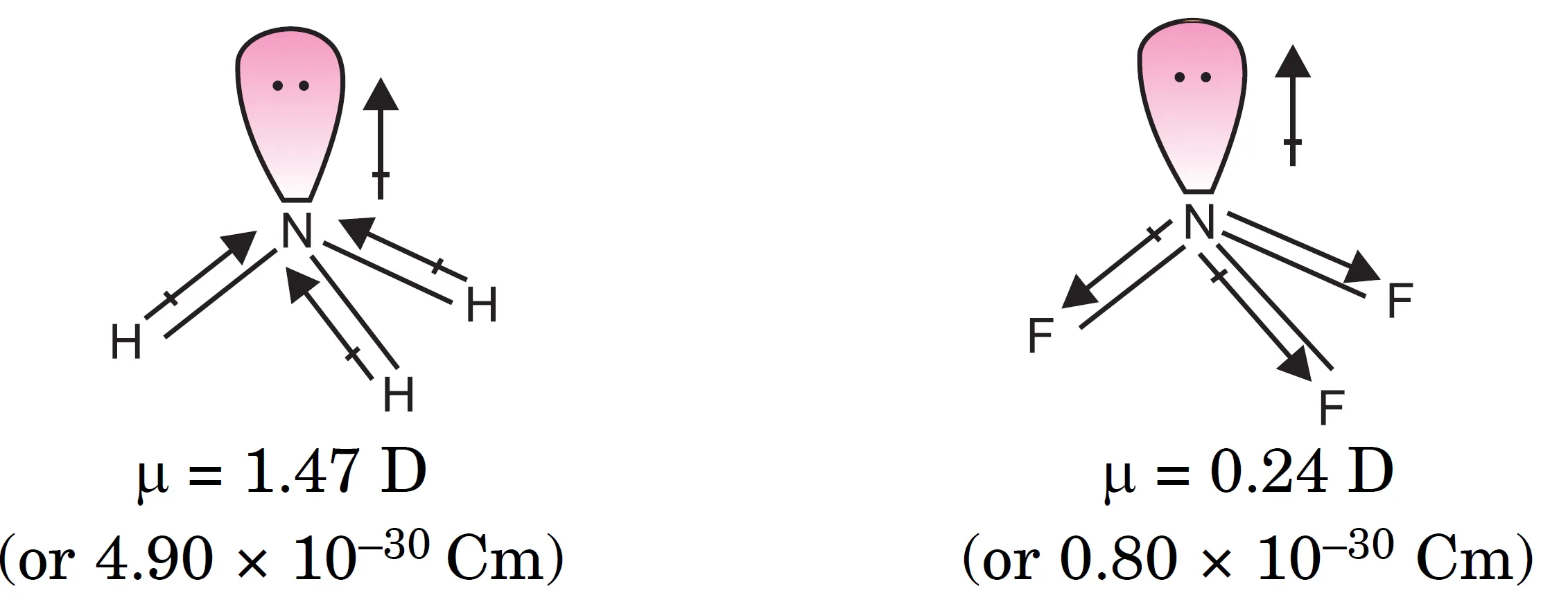
What Are the Dipole Moments of Some Common Molecules?
| Type of Molecule | Example | Dipole Moment μ (D) | Geometry |
|---|---|---|---|
| AB | HF | 1.78 | Linear |
| HCl | 1.07 | Linear | |
| HBr | 0.79 | Linear | |
| HI | 0.38 | Linear | |
| $H_2$ | 0 | Linear | |
| AB$_2$ | $H_2O$ | 1.85 | Bent |
| $H_2S$ | 0.95 | Bent | |
| $CO_2$ | 0 | Linear | |
| $BeF_2$ | 0 | Linear | |
| $CS_2$ | 0 | Linear | |
| AB$_3$ | $NH_3$ | 1.47 | Trigonal pyramidal |
| $NF_3$ | 0.24 | Trigonal pyramidal | |
| $BF_3$ | 0 | Trigonal planar | |
| AB$_4$ | $CH_4$ | 0 | Tetrahedral |
| $CHCl_3$ | 1.04 | Tetrahedral | |
| $CCl_4$ | 0 | Tetrahedral |
Short Answer Conceptual Type Questions (SAT)
Q1. Why does NH3 have a higher dipole moment than NF3 even though the N–F bond is more polar than the N–H bond?
Answer: In NH3, the lone pair dipole is in the same direction as the resultant dipole moment of the three N–H bonds, hence adding to it. In NF3, the lone pair dipole opposes the resultant dipole of the three N–F bonds, reducing the overall dipole moment.
Q2. What is the dipole moment of NH3 and NF3?
Answer: Dipole moment of NH3 = 1.47 D, while that of NF3 = 0.24 D.
Q3. Explain why CO2 is non-polar while H2O is polar although both are triatomic molecules.
Answer: CO2 is linear and the dipole moments of the two C=O bonds cancel each other, giving μ = 0 D. H2O has a bent geometry; the bond dipoles do not cancel and the net dipole moment is 1.85 D.
Q4. Why is the dipole moment of BF3 equal to zero?
Answer: BF3 has a symmetrical trigonal planar geometry. The three B–F bond dipoles cancel one another, giving a net dipole moment of zero.
Q5. Give one example of a polar molecule with bent geometry.
Answer: H2O or H2S.
Q6. Give one example of a non-polar tetrahedral molecule.
Answer: CH4 or CCl4.
Q7. Why is the dipole moment of CHCl3 not zero, while that of CCl4 is zero?
Answer: In CCl4, all C–Cl bond dipoles cancel due to symmetry, giving μ = 0 D. In CHCl3, the C–H bond is not identical to C–Cl bonds, so cancellation is incomplete, resulting in a net dipole moment of 1.04 D.
Q8. Arrange HF, HCl, HBr, HI in decreasing order of dipole moment.
Answer: HF ; (1.78 D) > HCl ; (1.07 D) > HBr ; (0.79 D) > HI ; (0.38 D).
Q9. What is the dipole moment of CO2 ? Explain.
Answer: μ = 0 D, because CO2 is linear and the bond dipoles cancel each other.
Q10. Compare the dipole moment of NH3 and NF3 with the help of electronegativity values.
Answer: In NH3, N (3.0) is more electronegative than H (2.1), so bond dipoles point towards N and add up with lone pair dipole. In NF3, F (4.0) is more electronegative than N (3.0), so bond dipoles point towards F, opposing the lone pair dipole, reducing net moment.
Multiple Choice Questions (MCQs)
Q1. Which of the following molecules has the highest dipole moment?
(a) HF
(b) HCl
(c) HBr
(d) HI
Answer: (a) HF
Q2. The dipole moment of CCl4 is zero because:
(a) All four C–Cl bonds are non-polar
(b) It has a linear geometry
(c) It has a tetrahedral geometry with symmetrical arrangement
(d) It has a trigonal planar geometry
Answer: (c)
Q3. Which of the following is polar?
(a) $BF_3$
(b) $CH_4$
(c) $CHCl_3$
(d) $CO_2$
Answer: (c) $CHCl_3$
Q4. Despite having more polar bonds, $NF_3$ has a lower dipole moment than $NH_3$ because:
(a) $N–F$ bonds are weaker than $N–H$ bonds
(b) Lone pair dipole cancels the resultant $N–F$ bond dipoles
(c) $NF_3$ has trigonal planar geometry
(d) Fluorine has less electronegativity
Answer: (b)
Assertion–Reason Questions
Q1.
Assertion (A): $NH_3$ has a higher dipole moment than $NF_3$.
Reason (R): In $NH_3$, the lone pair dipole and resultant bond dipoles act in the same direction, while in $NF_3$, they act in opposite directions.
- (a) Both A and R are true, and R is the correct explanation of A.
- (b) Both A and R are true, but R is not the correct explanation of A.
- (c) A is true, but R is false.
- (d) A is false, but R is true.
Answer: (a)
Q2.
Assertion (A): $CO_2$ has zero dipole moment.
Reason (R): The dipole moments of $C=O$ bonds cancel due to linear geometry.
- (a) Both A and R are true, and R is the correct explanation of A.
- (b) Both A and R are true, but R is not the correct explanation of A.
- (c) A is true, but R is false.
- (d) A is false, but R is true.
Answer: (a)
Q3.
Assertion (A): $CHCl_3$ has non-zero dipole moment.
Reason (R): The $C–H$ bond dipole cancels exactly the resultant dipole of $C–Cl$ bonds.
- (a) Both A and R are true, and R is the correct explanation of A.
- (b) Both A and R are true, but R is not the correct explanation of A.
- (c) A is true, but R is false.
- (d) A is false, but R is true.
Answer: (c)
Case Study
Read the passage and answer the following questions:
The molecules $NH_3$ and $NF_3$ both have pyramidal structures with one lone pair on nitrogen. In $NH_3$, the dipole moment is $1.47 , D$, whereas in $NF_3$ it is $0.24 , D$. This is due to the difference in how lone pair dipole interacts with bond dipoles. In $NH_3$, the lone pair adds to the bond dipoles, while in $NF_3$ it opposes them. Similarly, in polyatomic molecules like $CO_2$ and $H_2O$, geometry determines whether the dipole moment cancels or not.
Q1. What is the geometry of $NH_3$ and $NF_3$ molecules?
Answer: Both are trigonal pyramidal.
Q2. Why does $NH_3$ have a higher dipole moment than $NF_3$?
Answer: Because in $NH_3$, lone pair dipole and bond dipoles act in the same direction, while in $NF_3$ they oppose each other.
Q3. Give one example of a molecule with polar bonds but zero net dipole moment.
Answer: $CO_2$ or $BF_3$ or $CCl_4$.
Q4. State the dipole moment values of $NH_3$ and $NF_3$.
Answer: $NH_3$ = $1.47 , D$, $NF_3$ = $0.24 , D$.


