Chemistry plays an extremely vital role in everyday life. The principles of chemistry have benefited human life on a massive scale though we sometimes tend to overlook them. All textiles are processed by chemicals. Our clothes, some of them completely synthetic, involve processes, chemicals, dyes, and fibers. Consider cleanliness. Our household is filled with detergents, soaps, bleaches, kinds of toothpaste, etc. Moreover, even the food that we consume every day has several chemicals involved. Electronic materials, fuels, explosives, rocket propellants, building materials, etc., are all considered chemicals. Daily lives have been influenced by chemistry so much that one does not even realize that people come across chemicals at every moment; that humans themselves are beautiful chemical creations and all the activities are controlled by chemicals. Even in sickness and diseases, people resort to medicines, again chemicals.
Table of Contents
What are Drugs ?
Drugs are chemicals that alter or affect the physiology of a living system when taken in, they have a low molecular mass (~100 to 500u). These drugs produce a biological response when they interact with macromolecular targets. If the biological response is useful and therapeutic, we use them as medicines and are used for the diagnosis, prevention, and treatment of diseases. Medicines are the drugs that are legally administered by doctors when required under very small doses. Higher doses can be lethal and are potential poisons. Most of these interact with the nervous system, mostly the brain, to produce the required biological response. This usage of chemicals for a therapeutic effect is called chemotherapy.
Classification Of Drugs
Drugs are classified into various categories based on their pharmacological effect, drug action, chemical structure, and molecular targets. This classification is done to ensure that the drugs are prescribed and used efficiently and effectively. Let’s discuss each of these classifications in detail:
1]Based on their Pharmacological Effect:
Pharmacological effect refers to the effect that the drug has on the body. Based on this effect, drugs are classified into various categories:
a) Analgesics: Analgesics are drugs that relieve pain without causing loss of consciousness. They are further classified into two categories: non-narcotic analgesics (e.g., acetaminophen, ibuprofen) and narcotic analgesics (e.g., morphine, codeine).
b) Antacids: Antacids are drugs that neutralize stomach acid and relieve heartburn, acid indigestion, and upset stomach. Examples include calcium carbonate, magnesium hydroxide, and aluminum hydroxide.
c) Anti-anxiety drugs: Anti-anxiety drugs, also known as anxiolytics, are drugs that reduce anxiety and nervousness. Examples include diazepam, lorazepam, and alprazolam.
d) Antibiotics: Antibiotics are drugs that fight bacterial infections. Examples include penicillin, tetracycline, and erythromycin.
e) Antidepressants: Antidepressants are drugs that treat depression and other mood disorders. Examples include fluoxetine, sertraline, and paroxetine.
f) Antihistamines: Antihistamines are drugs that block the effects of histamine, a chemical produced by the body in response to allergens. They are used to treat allergies and relieve symptoms such as sneezing and itching. Examples include diphenhydramine, loratadine, and cetirizine.
g) Anti-inflammatory drugs: Anti-inflammatory drugs are drugs that reduce inflammation and swelling. They are used to treat conditions such as arthritis and asthma. Examples include aspirin, ibuprofen, and naproxen.
h) Antipsychotics: Antipsychotics are drugs that treat psychotic disorders such as schizophrenia. Examples include haloperidol, chlorpromazine, and clozapine.
i) Bronchodilators: Bronchodilators are drugs that relax the muscles around the airways in the lungs, making it easier to breathe. They are used to treat conditions such as asthma and chronic obstructive pulmonary disease (COPD). Examples include albuterol, salmeterol, and ipratropium.
2]Based on their Drug Action:
Drug action refers to how a drug works in the body to produce a therapeutic effect. Based on this action, drugs are classified into the following categories:
a) Agonists: Agonists are drugs that activate receptors in the body and produce a therapeutic effect. Examples include morphine (an opioid agonist) and albuterol (a beta-2 agonist).
b) Antagonists: Antagonists are drugs that block the action of agonists. Examples include naloxone (an opioid antagonist) and flumazenil (a benzodiazepine antagonist).
c) Enzyme inhibitors: Enzyme inhibitors are drugs that block the action of enzymes in the body. Examples include acetylcholinesterase inhibitors (used to treat Alzheimer’s disease) and angiotensin-converting enzyme (ACE) inhibitors (used to treat hypertension).
d) Ion channel blockers: Ion channel blockers are drugs that block the flow of ions through ion channels in the body. Examples include calcium channel blockers (used to treat hypertension) and sodium channel blockers (used to treat epilepsy).
3]Based On Chemical Structure
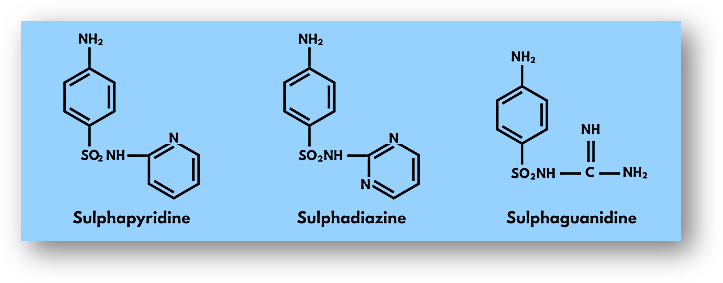
Drug classification based on chemical structure refers to the grouping of drugs based on their chemical composition and structure. This classification system is particularly useful for understanding the physical and chemical properties of drugs, as well as their mechanisms of action and potential side effects.
Here are some common classes of drugs based on their chemical structure:
Alcohols: Alcohols are compounds that contain a hydroxyl (-OH) group attached to a carbon atom. Ethanol, the active ingredient in alcoholic beverages, is an example of an alcohol that is used medicinally as a solvent and antiseptic.
Amines: Amines are compounds that contain a nitrogen atom that is bonded to one or more carbon atoms. Many drugs, including antidepressants, antihistamines, and muscle relaxants, are amines.
Carboxylic acids: Carboxylic acids are compounds that contain a carboxyl (-COOH) group attached to a carbon atom. Aspirin, a popular pain reliever and anti-inflammatory drug, is an example of a carboxylic acid.
Esters: Esters are compounds that are formed when an alcohol reacts with a carboxylic acid. Many drugs, including aspirin, are esters.
Phenols: Phenols are compounds that contain a hydroxyl (-OH) group attached to an aromatic ring. Many drugs, including antiseptics, disinfectants, and anesthetics, are phenols.
4] Base On Molecular Targets
Drug classification based on molecular targets refers to categorizing drugs based on their specific interactions with target molecules in the body, such as receptors, enzymes, and ion channels. Understanding these interactions can help in the development of new drugs and the identification of potential drug interactions and side effects. The following are some of the commonly recognized categories of drugs based on molecular targets:
G protein-coupled receptor (GPCR) agonists and antagonists: GPCRs are a family of cell surface receptors that mediate numerous physiological responses in the body, such as neurotransmission, hormone secretion, and immune response. Drugs that activate or inhibit GPCRs can have significant therapeutic effects, such as pain relief or reduced inflammation. Examples of GPCR agonists include morphine (used to relieve severe pain) and epinephrine (used to treat asthma). Examples of GPCR antagonists include beta blockers (used to treat hypertension) and antihistamines (used to treat allergies).
Enzyme inhibitors: Enzymes are molecules that catalyze biochemical reactions in the body, and their inhibition can prevent or modify the course of a disease. Enzyme inhibitors can be classified into two categories: irreversible inhibitors and reversible inhibitors. Irreversible inhibitors bind covalently to the enzyme, while reversible inhibitors bind non-covalently and can be either competitive or non-competitive. Examples of enzyme inhibitors include acetylcholinesterase inhibitors (used to treat Alzheimer’s disease) and protease inhibitors (used to treat HIV).
Conceptual Questions(FAQs)
Question 1: What are drugs?
Answer:
Drugs are chemicals that alter or affect the physiology of a living system when taken in. They have a low molecular mass (~100 to 500u). These drugs produce a biological response when they interact with macromolecular targets. If the biological response is useful and therapeutic, we use them as medicines and are used for the diagnosis, prevention and treatment of diseases.
Question 2: What are the various factors on which drugs are classified?
Answer:
Drugs can be classified on the basis of the following:
- Based on their pharmacological effect
- Based on their drug action
- Based on their chemical structure and
- Based on their molecular targets.
Question 3: Is caffeine a drug? Explain.
Answer:
Yes, Caffeine is a drug. It increases alertness by simulating the CNS.
Question 4: What are mind-altering drugs? Which is the most common mind-altering drug?
Answer:
Mind-altering drugs are various psychoactive substances used for their perception and mood altering effects. Alcohol is the most common mind-altering drug.
Question 5: What are opioids? Name the two strongest drugs.
Answer:
Opioids are drugs that act on opioid receptors and produce morphine-like effects. The strongest opioids are Oxymorphone and Hydromorphone.
Question 6: What is drug action? How are drugs classified according to this?
Answer:
Drug Action specifies how each drug generates a response from the organism.
For example, histamine causes inflammation in the body and all antihistamines inhibit the action of the given compound. There are several ways by which the action of histamine can be blocked.
Another example is that there are several medicines to reduce hypertension, but each type of drug has a different action and works in a different way.


