Anand Classes provides well-structured notes on the Characteristic Properties of Ionic Compounds for Class 11 Chemistry. Ionic compounds possess unique properties such as high melting and boiling points, solubility in polar solvents, electrical conductivity in molten or aqueous states, and crystalline solid structures. These detailed notes explain each property with examples to help students strengthen their understanding of ionic bonding. Perfect for board preparation and competitive exams, this study material is available in an easy-to-download PDF format. Click the print button to download study material and notes.
General Properties of Ionic Compounds
Ionic compounds exhibit several characteristic properties due to the electrostatic forces of attraction between their oppositely charged ions.
1. Physical State
- Ionic compounds usually exist as crystalline solids.
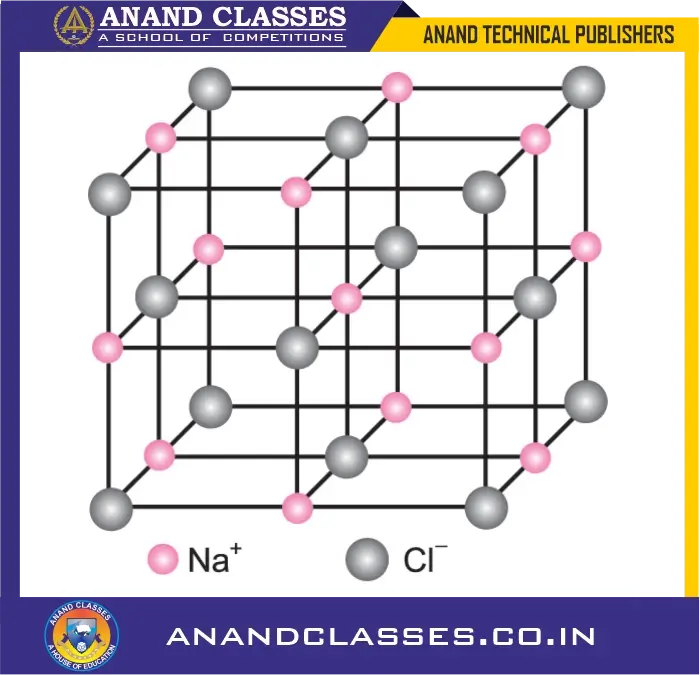
- X-ray studies reveal that they do not exist as independent molecules but as a three-dimensional lattice of ions.
- For example, in NaCl crystal, each Na$^+$ ion is surrounded by six Cl$^-$ ions and each Cl$^-$ ion is surrounded by six Na$^+$ ions.
- The number of oppositely charged ions present as the nearest neighbours around an ion is called its coordination number.
- In NaCl, the coordination number of both Na$^+$ and Cl$^-$ is 6.
- The geometric arrangement varies with ion size and magnitude of charges.
2. Melting and Boiling Points
- Ionic compounds have high melting and boiling points because of strong electrostatic forces holding the ions together.
- A large amount of energy is required to break the crystal lattice.
- The melting point depends on:
Charge on ions and Ionic radii
Closer ions ⇒ stronger attraction ⇒ higher melting point. - Example (sodium halides):
$$
\mathrm{NaF (1270\ K) > NaCl (1073\ K) > NaBr (1023\ K) > NaI (924\ K)}
$$
3. Solubility
- Ionic compounds are generally soluble in water and other polar solvents with high dielectric constants.
- This is because water molecules stabilize the ions by strong ion–dipole interactions.
- They are usually insoluble in non-polar solvents (e.g., benzene, ether).
4. Electrical Conductivity
- Molten or aqueous solutions of ionic compounds conduct electricity because ions are free to move.
- Solid crystals do not conduct electricity because the ions are fixed in the lattice and cannot move.
5. Ionic Reactions
- When dissolved in water, ionic compounds split into free ions.
- Their chemical reactions are characteristic of these ions and are known as ionic reactions.
- Such reactions are usually fast and instantaneous.
6. Non-directional Character
- The ionic bond is non-directional.
- Each ion is surrounded uniformly by oppositely charged ions in all directions.
- As a result, the electrical field is uniform and the bond does not show any directionality.
Short Answer Conceptual Types Questions (SAT) on Properties of Ionic Compounds
Q1. Why do ionic compounds have high melting and boiling points?
Because strong electrostatic forces of attraction between cations and anions require a large amount of energy to break the crystal lattice.
Q2. Why are ionic compounds generally soluble in water but insoluble in non-polar solvents?
Water is a polar solvent with a high dielectric constant, which stabilizes ions by ion–dipole interactions. Non-polar solvents cannot overcome the strong ionic forces.
Q3. Why do ionic compounds conduct electricity in molten or aqueous state but not in solid state?
In solid state, ions are fixed in the lattice and cannot move. In molten or solution state, ions are free to move, allowing conduction.
Q4. What is the coordination number in NaCl crystal?
Both Na$^+$ and Cl$^-$ ions have a coordination number of 6.
Multiple Choice Questions (MCQs) With Answers and Explanation on General Properties of Ionic Compounds
1. Ionic compounds in solid state are:
A) Good conductors of electricity
B) Poor conductors of electricity
C) Metallic in nature
D) Covalent in nature
Answer: B (Ions are not free to move in solid state)
2. Which of the following will have the highest melting point?
A) NaCl
B) NaBr
C) NaI
D) NaF
Answer: D (NaF, due to smaller ionic size and stronger attraction)
3. The coordination number of Cl$^-$ in NaCl crystal is:
A) 4
B) 6
C) 8
D) 12
Answer: B (Each Cl$^-$ is surrounded by 6 Na$^+$ ions)
Assertion Reason Type Questions With Answers and Explanation on General Properties of Ionic Compounds
Assertion (A): Ionic compounds are generally soluble in water.
Reason (R): Water has a high dielectric constant which reduces electrostatic forces between ions.
A) Both A and R are true, and R is the correct explanation of A
B) Both A and R are true, but R is not the correct explanation of A
C) A is true, R is false
D) A is false, R is true
Answer: A
Assertion (A): Solid NaCl does not conduct electricity.
Reason (R): NaCl contains only neutral molecules.
A) Both A and R are true, and R is the correct explanation of A
B) Both A and R are true, but R is not the correct explanation of A
C) A is true, R is false
D) A is false, R is true
Answer: C (NaCl contains ions but they are fixed in the crystal lattice)
Case Study based on General Properties of Ionic Compounds
Passage:
A student compares the melting points of NaF, NaCl, NaBr, and NaI. He observes that the melting points decrease in the order
$$
\mathrm{NaF > NaCl > NaBr > NaI}
$$
He also notes that solid NaCl does not conduct electricity but molten NaCl does.
Questions:
- Which compound has the strongest ionic bond?
- Explain the trend in melting points of sodium halides.
- Why does molten NaCl conduct electricity?
Answers:
- NaF (smallest ions, strongest electrostatic force)
- As the size of the halide ion increases from F$^-$ to I$^-$, lattice enthalpy decreases, lowering the melting point.
- In molten state, ions are free to move and carry electric current.


