In our daily lives, chemistry plays a significant role. Chemical molecules make up our most fundamental needs, such as shelter, food, clothing, and medication. Organic, analytical, physical, inorganic, and biological properties are all used in pharmaceutical chemistry. The knowledge of biological science is essential for understanding the method of action of pharmaceuticals, their effect and adverse effects on human body parts, as well as their reverse effects.
Table of Contents
Drugs – Target Interaction
The term ‘drug’ is derived from the French word ‘drogue,’ which means a dry herb, and is used in medicine. Plants still provide the basis for several medications. Nowadays, the majority of the pharmaceuticals we use are synthetic, created by scientists’ inventiveness. A drug is a substance used to diagnose, prevent, cure, or relieve the symptoms of a disease. Understanding the chemistry of drugs will assist us in comprehending their usage and abuse. Different people respond to these medications in different ways.
An ideal drug is one that does not disrupt physiological processes, is innocuous to the host yet kills hazardous organisms, is localized to the affected spot, and has the fewest adverse effects possible. The study of pharmaceutical chemistry is particularly significant for identification, preservation, finding various combinations of medications, expiry dates, storage conditions, and so on. Chemotherapy is a type of treatment that uses chemical chemicals to treat an illness.
Drugs are made from chemical substances collected from plants, minerals, animals, microorganisms, and other sources or synthesized in the lab. Drugs can be injected into the body as well as eaten orally.
Classification of Drugs
Drugs can be classified in a variety of ways, as shown below :
- Drug classification based on pharmacological effects: These drugs have an impact on biological functions such as digestion, blood circulation, and breathing. Analgesics, for example, are pain relievers; antacids, on the other hand, are used to relieve stomach pain and irritation; tranquillizers, on the other hand, are pharmaceuticals that impact the central nervous system; antibiotics and antiseptics, respectively, are used to prevent or destroy microorganisms.
- Drug classification based on the particular biochemical process: These drugs are intended to treat a specific ailment. Pain relievers, anti-arthritis drugs, local an-aesthetic agents, and other drugs with various biological mechanisms of action are examples. They stimulate or depress the central or peripheral nervous systems.
- Drug classification based on molecular targets: Target orientated drugs are those that interact with specific biomolecules. The drug interacts with biomolecules such as carbohydrates, proteins, nucleic acids, and other biomolecules. For medicinal, this classification, based on molecular targets is very important and useful.
- Drugs classification based on chemical structure: Alcohols, ketones, hydrocarbons, esters, amides, lactones, phenols, and other drugs are divided into many categories. Chemically, compounds with comparable chemical structures should have similar chemical properties, however, they do not have identical biological qualities. Amino alcohols, for example, do not all have the same biological function. As a result, classification based on drug effects is more precise.
Action of Drugs on Targets
Enzymes catalyze many biochemical reactions in our bodies, allowing them to occur faster while maintaining the same energy levels of the substrates. Without enzymes, the majority of our cells’ reactions would be too slow to keep us alive. Receptors are the components that make up the communication system. The receptors are where it all starts. The receptors are highly specialized macromolecules that react chemically with the drug and are found in the tissues. Many biological receptors are macromolecules that are made up of proteins, nucleic acids, lipids, and other components. To comprehend drug-target interactions, one must first comprehend substrate, enzyme, and drug interactions.
Enzyme as a drug target
Let’s start with an overview of enzymes and how they work. Enzymes are protein-like compounds that exist in the human body. Their primary role is to act as biological catalysts in the body’s chemical reactions. They control these chemical reactions but are unaffected by them themselves. Substrates are compounds that bind to enzymes. New molecules, known as products, will be produced as a result of the reaction. The substrate is tightly held in the active site of the enzymes. As a result, the reagent is able to successfully react with the substrate. Hydrogen bonding, ionic bonding, Van der Waal forces, and other interactions help the enzyme hold the substrate in the active site. The enzyme’s other role is to provide functional groups that will react with substrates to carry out the chemical reactions that are required to sustain all of our life activities.
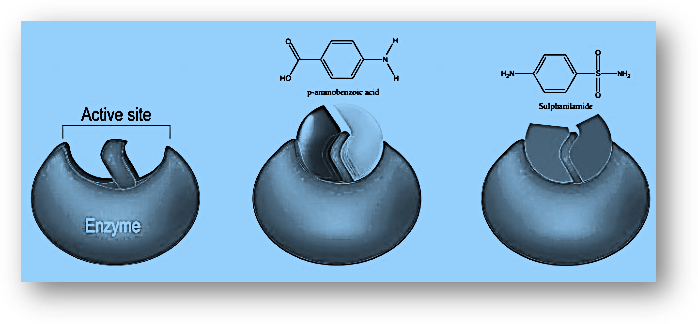
Enzyme Catalytic Action
Different processes are catalyzed by enzymes. The enzyme’s first duty is to make contact with the substrate. Active sites in enzymes are where the substrate is bound. Hydrogen bonding, ionic contact, and van der Waal’s forces all play a role in the interaction between the substrate and the enzyme. The enzyme’s second role is to provide functional groups that will attack the substrate and catalyze the reaction.
Drug Enzyme Interaction
Enzyme actions must be controlled at times, and we do so via enzyme inhibitors. The enzyme will be the pharmacological target in this case, with the medication attempting to obstruct the enzyme’s function. There are two ways to accomplish this:
- By attaching themselves to the enzyme’s active site, some drugs will compete with the substrate. Competitor inhibitors are what they’re called. The substrate will not be able to connect to the enzyme in this case, and the reaction will not take place. An approach like this will not function if the substrate concentration is much higher than the drug concentration.
- Allosteric inhibitors will then bind themselves to the enzymes’ allosteric sites. This is a site that is not currently functioning. They will alter the enzyme’s shape and structure as a result of this. The substrate is no longer able to recognize the enzyme and will not bind to the active site, preventing the catalytic activity from taking place.
Receptors act as drug targets
Our bodies have receptors, which are proteins. Their major job is to help neurons communicate with each other and with muscles. These biomolecules aid communication by allowing humans to communicate through chemical messengers, which are specialized substances.
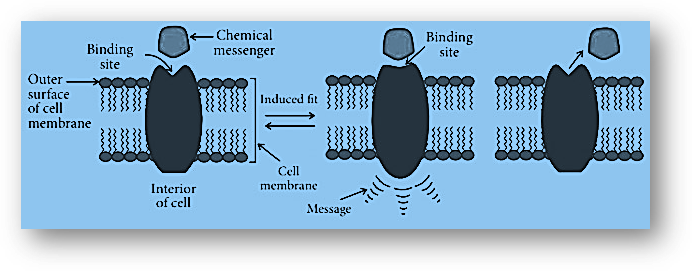
Cell membranes are normally where receptors are present. In an unusual fashion, they are embedded in the cell membrane. The membrane encapsulates the majority of their body. In the area outside the cell, only a small piece of the molecule protrudes from the membrane. The active site of the receptor is located in this protruding area.
As a result, when a chemical messenger approaches a receptor, it binds to the receptor’s active region, causing the molecule’s shape to change. Inside the cell’s membrane, this will send the message. As a result, without the chemical messenger even entering the cell, the message will be relayed to the cell.
In the human body, there are many different types of receptors. Many chemical messengers interact with these receptors. The active sites of the receptors differ in shape, structure, and chemical makeup, allowing them to recognize their specific message. This enables for selective interactions between receptors and messengers. Drugs that target these receptors work by interfering with their normal activity. They attach to their active site, inhibiting their actions and preventing the message from being communicated. Antagonists, such as naltrexone and naloxone, are two examples. A drug can also target receptors by imitating natural messengers. This activates the receptors, which results in a physiologic response. Agonists are medications that cause receptors to respond in a positive way.
Sample Questions
Question 1: What is a Drug target?
Answer:
Any entity that is targeted by a drug to affect its behavior or function is referred to as a drug target.
Question 2: Which enzyme location is known as an allosteric site?
Answer:
The enzyme location, which is not the active site, where some drugs blind to an enzyme.
Some drugs bind to an enzyme at an allosteric site, which is different from the active site. As a result, the enzyme’s structure changes, making it more difficult for the substrate to recognise the enzyme’s active site. Noncompetitive inhibition is what this means.
Question 3: How drugs can stop enzymes from catalyzing by binding to their catalytic sites.
Answer:
Drugs that target enzymes can assault the enzyme’s active site as well as its allosteric location. By preventing the substrate from attaching to either of the sites, it reduces the enzymes’ catalytic activity.
Question 4: What are receptors, and what distinguishes them from enzymes?
Answer:
In the body, biological macromolecules provide a variety of roles. Enzymes, for example, are proteins that act as biological catalysts in the body; receptors, on the other hand, are proteins that are essential for the body’s communication system.
Question 5: What is a Biological target?
Answer:
Any biological entity that is targeted for modification is referred to as a biological target.


