Anand Classes Notes explains across any period in the periodic table, atomic radius generally decreases from left to right. This trend occurs because as the atomic number increases, the nuclear charge grows while electrons are added to the same principal energy level. Without significant shielding from electrons in the same shell, the increased effective nuclear pull draws electrons closer to the nucleus, resulting in a smaller atomic size.
Table of Contents
Variation of Atomic Radius in a Period
In general, atomic radii decrease with an increase in atomic number when moving from left to right across a period in the periodic table.
For example, in the second period, the atomic radii decrease from lithium (Li) to neon (Ne) through beryllium (Be), boron (B), carbon (C), nitrogen (N), oxygen (O), and fluorine (F), as shown in Table 1.
Table 1: Decrease in Atomic Radii in the Second Period
| Element (Atomic No.) | Nuclear Charge | Outer Electronic Configuration | Atomic Radius (pm) |
|---|---|---|---|
| ³Li | +3 | 2s¹ | 152 |
| ⁴Be | +4 | 2s² | 111 |
| ⁵B | +5 | 2s² 2p¹ | 88 |
| ⁶C | +6 | 2s² 2p² | 77 |
| ⁷N | +7 | 2s² 2p³ | 70 |
| ⁸O | +8 | 2s² 2p⁴ | 74 |
| ⁹F | +9 | 2s² 2p⁵ | 72 |
| ¹⁰Ne* | +10 | 2s² 2p⁶ | 160* |
** Note : For neon, the value given is the van der Waals radius (inert gas radius).
The variation in atomic radius with atomic number for the second period can be explained based on the increasing nuclear charge across the period. As the atomic number increases from lithium to fluorine, the nuclear charge increases progressively by one unit. The additional electrons are added to the same principal shell (n = 2).
Since electrons in the same shell do not significantly shield one another from the nucleus, the increased nuclear charge is not neutralized by the extra valence electrons. As a result, the outer electrons are pulled closer to the nucleus by the greater effective nuclear charge, causing the atomic size to decrease steadily across the period.
Anomalous Behaviour of Oxygen and Fluorine
Why atomic radius of oxygen is slightly more than Nitrogen ?
From Li to N, the atomic radius decreases regularly. However, after nitrogen, the atomic radius increases slightly for oxygen and then decreases again for fluorine.
- Nitrogen: All three 2p orbitals have one electron each (2s² 2px¹ 2py¹ 2pz¹).
- Oxygen: One of the 2p orbitals has two electrons (2s² 2pₓ² 2pᵧ¹ 2pz¹). The paired electrons in the same orbital experience increased interelectronic repulsion, which outweighs the effect of the increased nuclear charge, resulting in a slightly larger size than nitrogen.
- Fluorine: Two of the 2p orbitals have paired electrons, but here the increased nuclear charge outweighs the repulsions, so the size decreases again compared to oxygen.
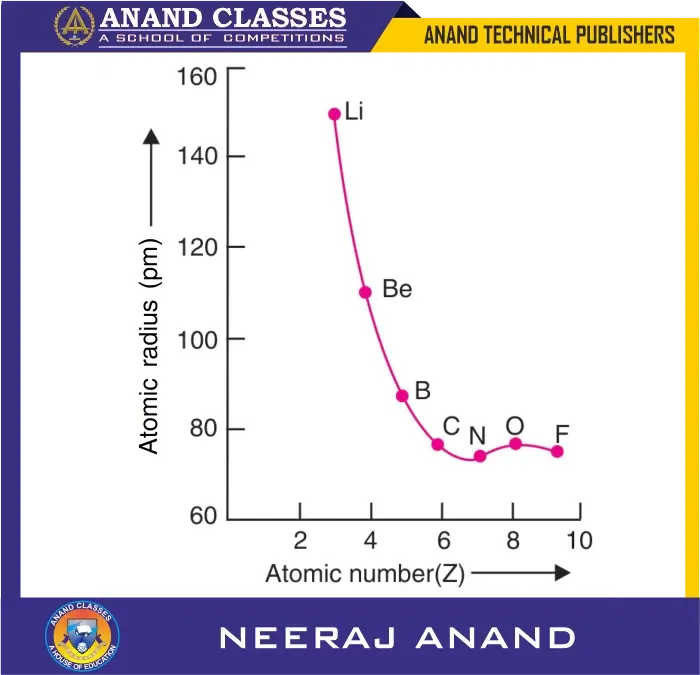
across the second period
It is notable that both oxygen and fluorine have atomic sizes larger than nitrogen due to this interplay of nuclear charge and electron–electron repulsion.
Variation of Atomic Radius in Third Period
A similar trend is observed in the third period, as shown in Table.2.
Table 2: Decrease in Atomic Radii in the Third Period
| Element (Atomic No.) | Atomic Radius (pm) |
|---|---|
| ¹¹Na | 186 |
| ¹²Mg | 160 |
| ¹³Al | 143 |
| ¹⁴Si | 118 |
| ¹⁵P | 110 |
| ¹⁶S | 104 |
| ¹⁷Cl | 99 |
Conclusion
Except for noble gases:
- Alkali metals (extreme left) have the largest atomic size in a period.
- Halogens (extreme right) have the smallest atomic size in a period.
- Atomic size generally decreases from left to right across a period.
FAQs – Variation of Atomic Radius in a Period
Q1. Why does atomic radius decrease across a period?
As we move from left to right in a period, the number of protons in the nucleus increases. This leads to a stronger nuclear charge pulling electrons closer to the nucleus. Since electrons are added to the same energy level, shielding remains almost constant, resulting in a decrease in atomic size.
Q2. Which element in a period has the smallest atomic radius?
The noble gas at the end of the period usually has the smallest atomic radius because it has the highest effective nuclear charge among the elements in that period.
Q3. Does the shielding effect change across a period?
Only slightly. Electrons are added to the same shell, so shielding effect does not increase significantly. The increase in nuclear charge dominates, causing the atomic radius to shrink.
Q4. Are there any exceptions to the trend of decreasing atomic radius?
Yes, slight anomalies exist due to electron-electron repulsion in half-filled and fully filled orbitals. For example, oxygen has a slightly larger radius than nitrogen because of extra electron repulsion in the paired electrons of the 2p orbital.
Q5. How does this trend differ from variation down a group?
Across a period, atomic radius decreases due to increasing nuclear charge. Down a group, atomic radius increases because new electron shells are added, outweighing the effect of increased nuclear charge.
🔍 Do You Know? – Atomic Radius in a Period
- The term “atomic radius” doesn’t have a fixed definition — it depends on how we measure it (covalent radius, metallic radius, van der Waals radius).
- Fluorine has one of the smallest atomic radii among all elements due to its high nuclear charge and strong attraction for electrons.
- Even though noble gases are placed at the end of a period, their van der Waals radius is actually larger than halogens because they don’t form covalent bonds — but in periodic trends, we generally consider covalent radius, which is smaller.
- The first period shows the largest relative decrease in atomic radius compared to later periods because the effect of increasing nuclear charge is most prominent in small atoms.
- Transition elements show a smaller change in atomic radius across their series because the additional electrons go into inner d-orbitals, partially offsetting nuclear charge effects.
For premium study materials specially designed for JEE, NEET, NDA, and CBSE/ICSE Classes, visit our official study material portal:
👉 https://anandclasses.net.in/
To enroll in our offline or online coaching programs, visit our coaching center website:
👉 https://anandclasses.co.in/
📞 Call us directly at: +91-94631-38669
💬 WhatsApp Us Instantly
Need quick assistance or want to inquire about classes and materials?
📲 Click below to chat instantly on WhatsApp:
👉 Chat on WhatsApp
🎥 Watch Video Lectures
Get access to high-quality video lessons, concept explainers, and revision tips by subscribing to our official YouTube channel:
👉 Neeraj Anand Classes – YouTube Channel


