Anand Classes presents detailed Class 11 Chemistry notes on the Shapes of Sulphur Dioxide (SO₂), Ammonia (NH₃), and Water (H₂O) Molecules using VSEPR Theory. Learn how lone pairs and bond pairs affect molecular geometry and bond angles, making SO₂ a bent molecule, NH₃ a trigonal pyramidal molecule, and H₂O a bent molecule with higher bond angle distortion. These notes include diagrams, solved Q&A, and conceptual MCQs designed for NEET, JEE, and CBSE board exams. Perfect for quick revision and exam practice. Click the print button to download study material and notes.
What is the Shape of Molecules Containing Three Electron Pairs (AB3 or AB2L)?
If the valence shell of an atom contains three electron pairs, then the molecule has trigonal planar geometry (e.g., BF3).
However, if it contains one lone pair in addition to two bond pairs, the geometry gets distorted. For example, a molecule of the type AB2L (where L represents a lone pair), has V-shaped geometry as discussed for SO2 molecule.
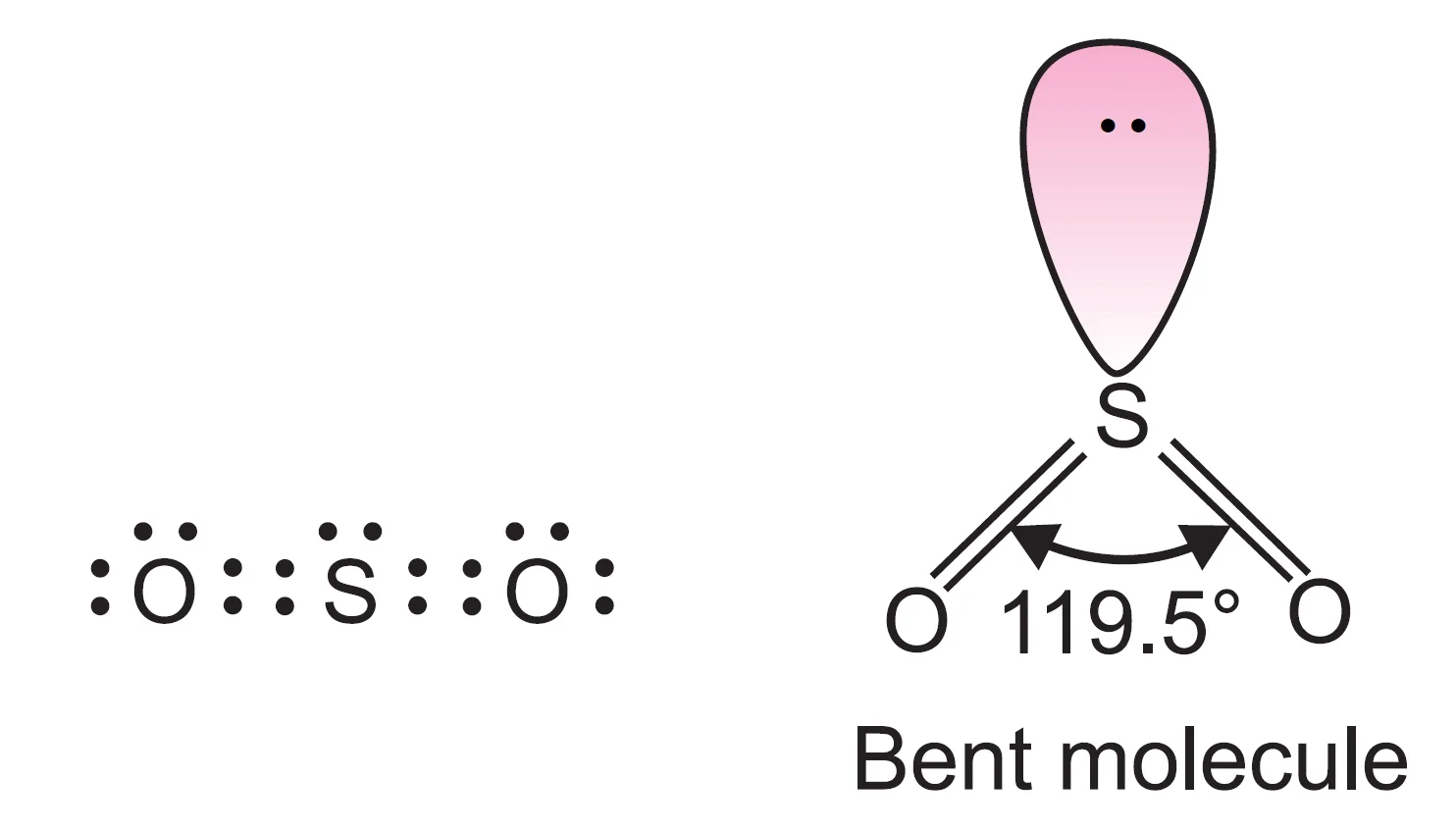
What is the Shape of Sulphur Dioxide (SO2) Molecule?
In SO2 molecule, there are three electron pairs (two bond pairs and one lone pair).
The three electron pairs should acquire a trigonal planar arrangement with bond angle 120o. Since one of the positions is occupied by a lone pair, the geometry may be described as angular, V-shaped, or bent shape.
Now, lone pair–bond pair repulsion is more than bond pair–bond pair repulsion. Therefore, bonded pairs of electrons are pushed closer, and the O–S–O bond angle gets reduced to 119.5o from 120o.
What is the Shape of Molecules Containing Four Electron Pairs (AB4, AB3L, AB2L2)?
As already learnt, the molecule AB4 has tetrahedral geometry. But if lone pairs are also present in addition to bond pairs, the geometry gets distorted.
This may be illustrated by taking two examples:
(a) Molecules containing 3 bond pairs and 1 lone pair (AB3L), e.g., NH3
(b) Molecules containing 2 bond pairs and 2 lone pairs (AB2L2, e.g., H2O
What is the Shape of Ammonia (NH3) Molecule?
In ammonia (NH3) molecule, the central nitrogen atom (Z = 7, 1s2 2s2 2p3) has five valence electrons. Three of these valence electrons share electrons with three hydrogen atoms forming three bond pairs around the nitrogen atom. The remaining two electrons are present as a lone pair.
Thus, in ammonia, nitrogen is surrounded by four electron pairs (three bond pairs and one lone pair). These four electron pairs adopt tetrahedral geometry. But all the four electron pairs around nitrogen are not equivalent (three bond pairs and one lone pair), and therefore, ammonia has distorted tetrahedral geometry.
The bond angle is not 109.5o but 107o. This distortion is due to the presence of one lone pair in addition to bond pairs. As already explained, lone pair–bond pair repulsion is more than bond pair–bond pair repulsion. As a result, the lone pair repels the bond pairs strongly and decreases the bond angle to 107o.
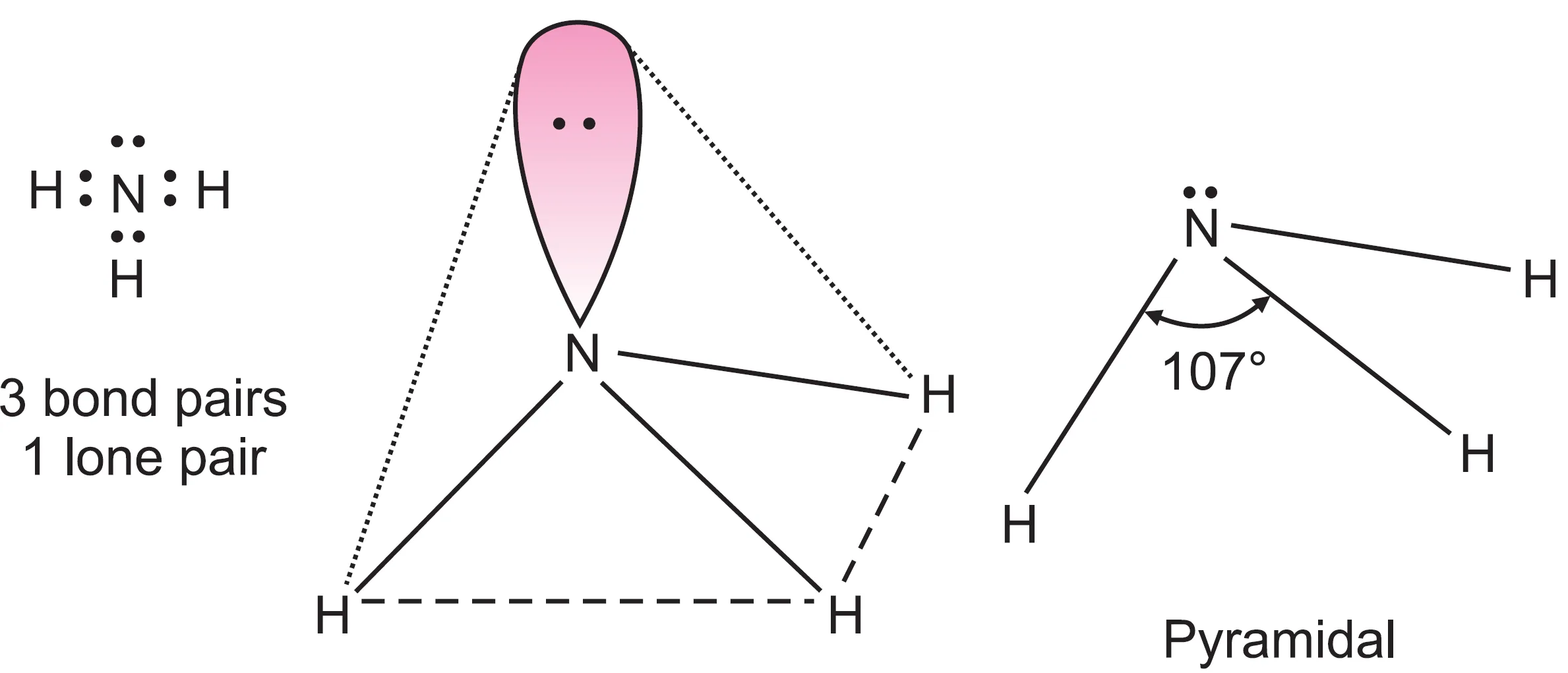
The geometry of ammonia molecule is also regarded as pyramidal. Other molecules with the same shape are PCl3, NF3, H3O+, etc.
What is the Shape of Water (H2O) Molecule?
In the case of water (H2O) molecule, the central oxygen atom (Z = 8, 1s2 2s2 2p4) has six valence electrons. In the formation of water molecule, the electrons form two bond pairs with two hydrogen atoms, leaving four electrons as two lone pairs.
The four electron pairs around the central oxygen atom adopt tetrahedral geometry. However, all the four electron pairs around O are not equivalent, and therefore, geometry is distorted tetrahedral.
The bond angle in water molecule is not 109.5o but 104.5o. The distortion is due to the presence of two lone pairs in addition to bond pairs.
The repulsion order is:
lone pair–lone pair > lone pair–bond pair > bond pair–bond pair
Thus, in water, the two lone pairs move away from each other, while the two O–H bonds are forced closer together (even more than N–H bonds in NH3). This decreases the H–O–H bond angle to 104.5o.
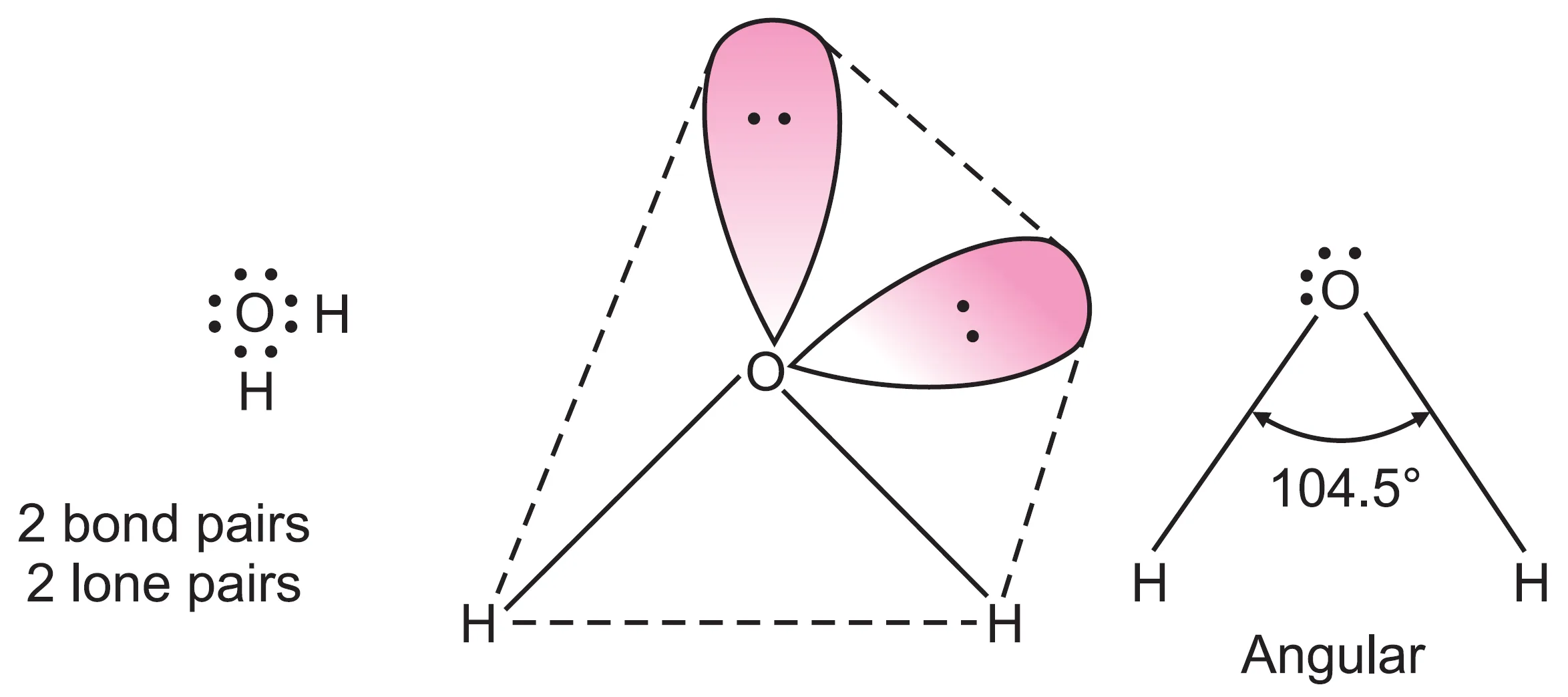
The resulting geometry is regarded as bent or angular. Other molecules with the same shape are H2S, F2O, SCl2, etc.
Why Do CH4, NH3, and H2O Have Different Geometries Despite Having Four Electron Pairs?
The central atoms (C, N, and O) in CH4, NH3, and H2O all have four electron pairs around them. Therefore, these molecules adopt tetrahedral geometries.
- In methane (CH4), there are no lone pairs → geometry is regular tetrahedral (109.5o).
- In ammonia (NH3), there is one lone pair → geometry is distorted tetrahedral or pyramidal (107o).
- In water (H2O), there are two lone pairs → geometry is further distorted tetrahedral or bent (104.5o).
Thus, the presence of lone pairs causes distortions due to greater lone pair–bond pair and lone pair–lone pair repulsions compared to bond pair–bond pair repulsions.
Short Answer Conceptual Type Questions (SAT)
Q1. Why does $SO_{2}$ molecule have a bent shape instead of trigonal planar geometry?
Answer: Because $SO_{2}$ has three electron pairs (two bond pairs and one lone pair). The lone pair causes greater lone pair–bond pair repulsion, pushing the $O–S–O$ bonds closer. Hence, the geometry becomes V-shaped (119.5°) instead of perfect trigonal planar (120°).
Q2. Why is the bond angle in $NH_{3}$ smaller than that in $CH_{4}$?
Answer: In $CH_{4}$, there are only bond pairs → bond angle $109.5^{\circ}$. In $NH_{3}$, there is one lone pair. Lone pair–bond pair repulsion > bond pair–bond pair repulsion, reducing the bond angle to $107^{\circ}$.
Q3. Explain why the $H–O–H$ bond angle in water is smaller than the $H–N–H$ bond angle in ammonia.
Answer: In $H_{2}O$, there are two lone pairs. The repulsion order is:
$$ \text{lone pair–lone pair} > \text{lone pair–bond pair} > \text{bond pair–bond pair} $$
Two lone pairs repel the bond pairs more strongly, compressing the bond angle to $104.5^{\circ}$, smaller than $107^{\circ}$ in $NH_{3}$.
Multiple Choice Questions (MCQs) With Answers and Explanation
Q1. The bond angle in $SO_{2}$ is:
(a) $120^{\circ}$
(b) $109.5^{\circ}$
(c) $119.5^{\circ}$
(d) $104.5^{\circ}$
Answer: (c) $119.5^{\circ}$
Explanation: Ideal trigonal planar angle is $120^{circ}$, but the presence of a lone pair reduces it slightly to $119.5^{circ}$.
Q2. Which of the following molecules has pyramidal geometry?
(a) $CH_{4}$
(b) $NH_{3}$
(c) $H_{2}O$
(d) $SO_{2}$
Answer: (b) $NH_{3}$
Explanation: $NH_{3}$ has 3 bond pairs and 1 lone pair → distorted tetrahedral geometry, also called pyramidal.
Q3. Which has the smallest bond angle?
(a) $CH_{4}$
(b) $NH_{3}$
(c) $H_{2}O$
(d) $SO_{2}$
Answer: (c) $H_{2}O$
Explanation: $CH_{4}$ → $109.5^{\circ}$, $NH_{3}$ → $107^{\circ}$, $SO_{2}$ → $119.5^{\circ}$, $H_{2}O$ → $104.5^{\circ}$ (smallest).
Assertion-Reason Type Questions With Answers and Explanation
Q1.
Assertion (A): $NH_{3}$ has a bond angle of $107^{\circ}$.
Reason (R): Lone pair–bond pair repulsion is stronger than bond pair–bond pair repulsion.
Answer: Both Assertion and Reason are true, and Reason is the correct explanation.
Q2.
Assertion (A): Water ($H_{2}O$) molecule is angular with bond angle $104.5^{\circ}$.
Reason (R): The repulsion between two lone pairs on oxygen decreases the bond angle.
Answer: Both Assertion and Reason are true, and Reason is the correct explanation.
Q3.
Assertion (A): $SO_{2}$ is linear in shape.
Reason (R): It has three regions of electron density around the central atom.
Answer: Assertion is false, Reason is true.
Explanation: $SO_{2}$ has 3 regions, but one is a lone pair → bent, not linear.
Case Study Based Questions
Read the following passage and answer the questions:
Case Study:
The central atoms in $CH_{4}, NH_{3},$ and $H_{2}O$ all have four electron pairs. $CH_{4}$ has no lone pair and is tetrahedral ($109.5^{\circ}$). $NH_{3}$ has one lone pair, making it pyramidal with bond angle $107^{\circ}$. $H_{2}O$ has two lone pairs, making it bent with bond angle $104.5^{\circ}$. The distortion is explained by the repulsion order:
$$ \text{lone pair–lone pair} > \text{lone pair–bond pair} > \text{bond pair–bond pair} $$
Questions:
- Why does $CH_{4}$ have a regular tetrahedral geometry?
Answer: Because it has 4 bond pairs and no lone pairs → all repulsions are equal. - Why is the bond angle in $NH_{3}$ smaller than in $CH_{4}$?
Answer: Due to lone pair–bond pair repulsion compressing the bond angle from $109.5^{\circ}$ to $107^{\circ}$. - Which molecule has the greatest distortion from tetrahedral geometry?
Answer: $H_{2}O$ (bond angle $104.5^{\circ}$). - Arrange $CH_{4}, NH_{3},$ and $H_{2}O$ in increasing order of bond angle.
Answer: $H_{2}O$ ($104.5^{\circ}$) < $NH_{3}$ ($107^{\circ}$) < $CH_{4}$ ($109.5^{\circ}$).


