Anand Classes provides detailed notes on the Rules for Writing Lewis Structures of Covalent Compounds for Class 11 Chemistry students. These rules include counting valence electrons, identifying the central atom, arranging bonds, completing octets, and ensuring stability of the molecule. Step-by-step examples like CH4, CO, and NO2– are explained to help students master the concept for board exams as well as JEE and NEET preparation. Click the print button to download study material and notes.
Rules for Writing Lewis Structures of Covalent Compunds
The following steps are adopted for writing the Lewis dot structures (Lewis structures):
Step 1. Calculate the total number of valence electrons by adding the valence electrons of all combining atoms.
Example: In methane (CH4), there are 8 valence electrons (4 from carbon and 4 from four H atoms).
Step 2. For anions, each negative charge means addition of one electron; for cations, each positive charge means removal of one electron.
Step 3. Guess the skeletal structure of the compound and distribute the total number of electrons as bonding shared pairs between the atoms.
Step 4. In general, the least electronegative atom occupies the central position. (Hydrogen and fluorine always occupy terminal positions.)
Step 5. After placing shared pairs as single bonds, the remaining electrons are either used to form multiple bonds or placed as lone pairs.
The basic requirement is that each bonded atom attains an octet configuration (except H, which requires only 2 electrons).
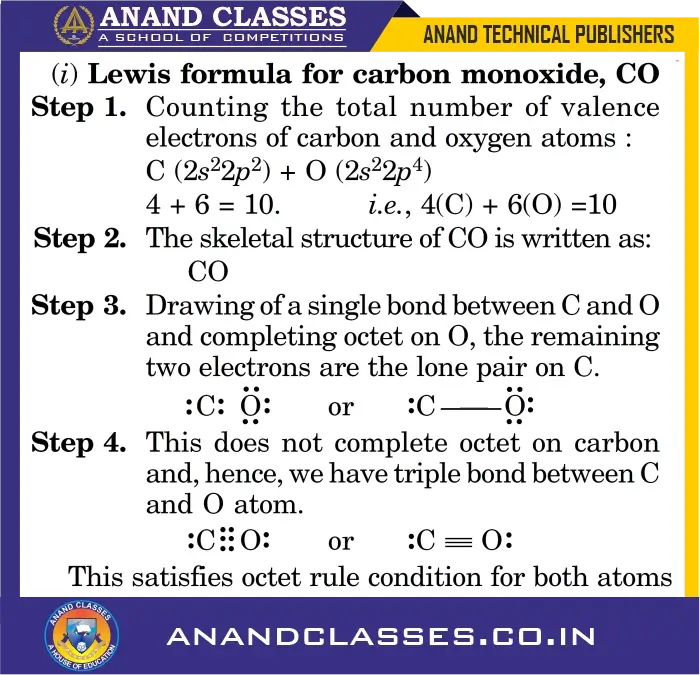
Example (i) Carbon Monoxide (CO)
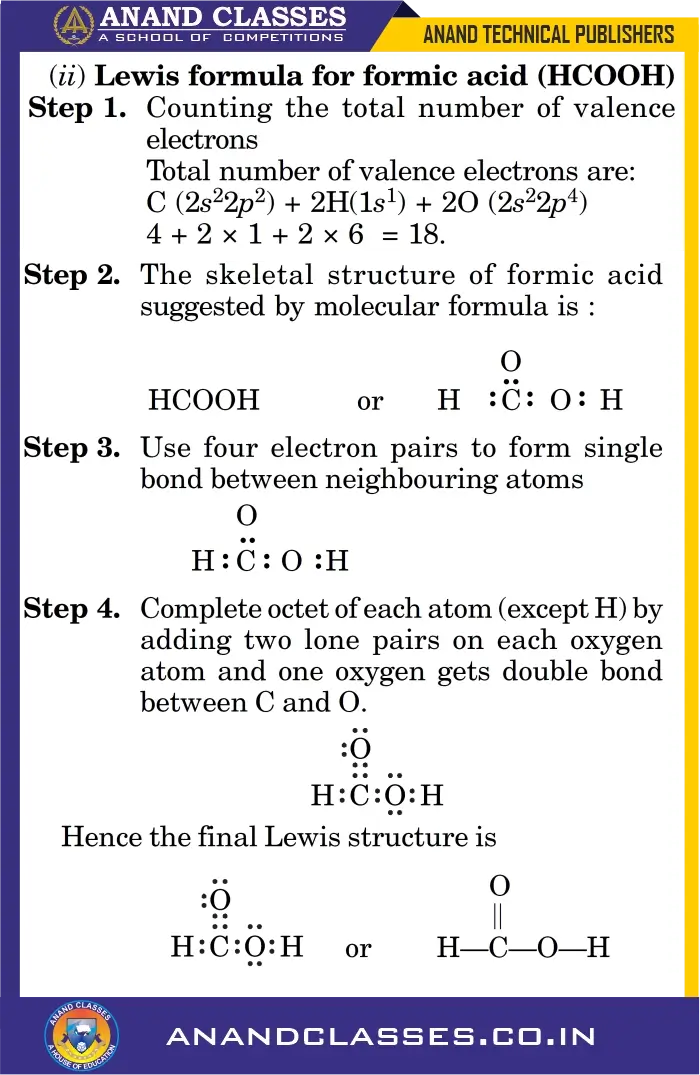
Example (ii) Formic Acid (HCOOH)
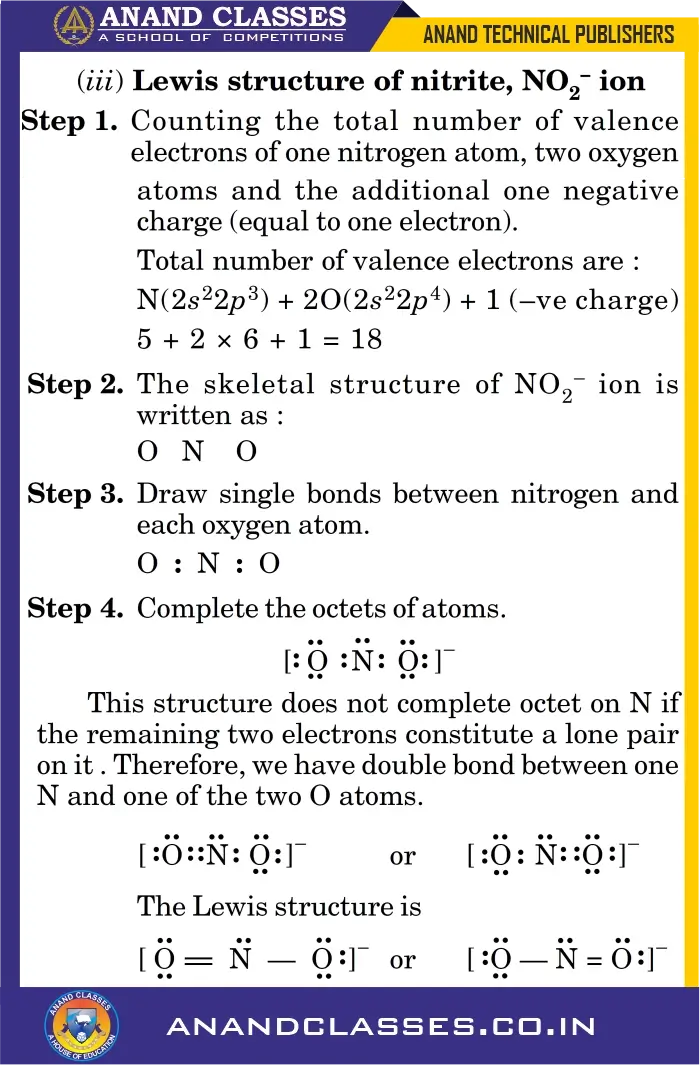
Example (iii) Nitrite Ion (NO2–)
Short Answer Conceptual Types Questions (SAT) on Lewis Structures of Covalent Compunds
Q1. What is a Lewis structure?
A Lewis structure is a diagram showing how valence electrons are arranged among atoms in a molecule or ion using dots (for electrons) and lines (for bonds).
Q2. Which atom is placed at the center of a Lewis structure?
Generally, the least electronegative atom is placed at the center (except H and F, which are always terminal).
Q3. What is the Octet Rule?
Atoms tend to share, gain, or lose electrons to attain 8 electrons in their valence shell (hydrogen follows the duplet rule).
Q4. Why does CO have a triple bond in its Lewis structure?
A triple bond is required because a single or double bond does not satisfy the octet rule for both C and O.
Q5. What is resonance in Lewis structures?
When two or more valid Lewis structures can be drawn for a molecule/ion (e.g., $NO_2^-$, $CO_3^{2-}$), the actual structure is a resonance hybrid of them.
Multiple Choice Questions (MCQs) With Answers and Explanation on Lewis Structures of Covalent Compunds
Q1. Which of the following molecules has a triple covalent bond?
(a) $CO_2$
(b) $N_2$
(c) $H_2O$
(d) $CH_4$
Answer: (b) $N_2$
Q2. Total number of valence electrons in $NO_2^-$ is:
(a) 16
(b) 17
(c) 18
(d) 24
Answer: (c) 18
Q3. In the Lewis structure of formic acid ($HCOOH$), carbon is bonded to:
(a) 2 hydrogens and 2 oxygens
(b) 1 hydrogen and 2 oxygens
(c) 1 hydrogen and 1 oxygen
(d) 2 oxygens only
Answer: (b) 1 hydrogen and 2 oxygens
Assertion Reason Type Questions With Answers and Explanation on Lewis Structures of Covalent Compunds
Q1.
Assertion (A): In carbon monoxide, C and O are connected by a triple bond.
Reason (R): Both atoms need to complete their octet.
- (a) A and R are true, R is the correct explanation of A
- (b) A and R are true, R is not the correct explanation of A
- (c) A is true, R is false
- (d) A is false, R is true
Answer: (a)
Q2.
Assertion (A): Hydrogen always occupies the central position in Lewis structures.
Reason (R): Hydrogen has only one valence electron.
- (a) A and R are true, R is the correct explanation of A
- (b) A and R are true, R is not the correct explanation of A
- (c) A is true, R is false
- (d) A is false, R is true
Answer: (d)
Case Study based on Lewis Structures of Covalent Compunds
Case:
A student is asked to draw the Lewis structure of $CO_2$. He calculates the total valence electrons as 16 (4 from C and 6 from each O). He places carbon in the center and attaches two oxygens with single bonds. After completing octets, the structure looks like:
O—C—O
with two lone pairs on each O.
Questions:
- Why is this structure incorrect?
- Draw the correct Lewis structure of $CO_2$.
- How many bonding pairs and lone pairs are present in the final structure?
Answers:
- The structure is incorrect because carbon does not complete its octet.
- Correct structure:
O = C = O
- Bonding pairs = 4 (two double bonds), Lone pairs = 4 (two on each oxygen).


