Anand Classes provides NCERT Solutions for Class 11 Chemistry Chapter – Chemical Bonding and Molecular Structure (Questions 4.11, 4.12, 4.13, 4.14, 4.15, 4.16, 4.17, 4.18, 4.19, 4.20) with detailed step-by-step explanations, diagrams, and conceptual clarity. These questions cover important topics like VSEPR Theory, Hybridisation (sp, sp², sp³, sp³d, sp³d²), Molecular Orbital Theory (MOT), Bond Order, Polarity, and Magnetic Properties of Molecules. All solutions are prepared according to the latest NCERT syllabus and CBSE guidelines, making them extremely useful for Board Exams, JEE, and NEET Chemistry preparation. Students can easily view or download the PDF for offline study. Click the print button to download study material and notes.
Q : Explain the Important Aspects of Resonance with Reference to the CO32- Ion?
Answer:
When a molecule cannot be represented by a single Lewis structure but its actual properties can be described by two or more contributing structures, the true structure is called a resonance hybrid of these structures.
For example, the carbonate ion (CO32-) can be represented by the following structure:
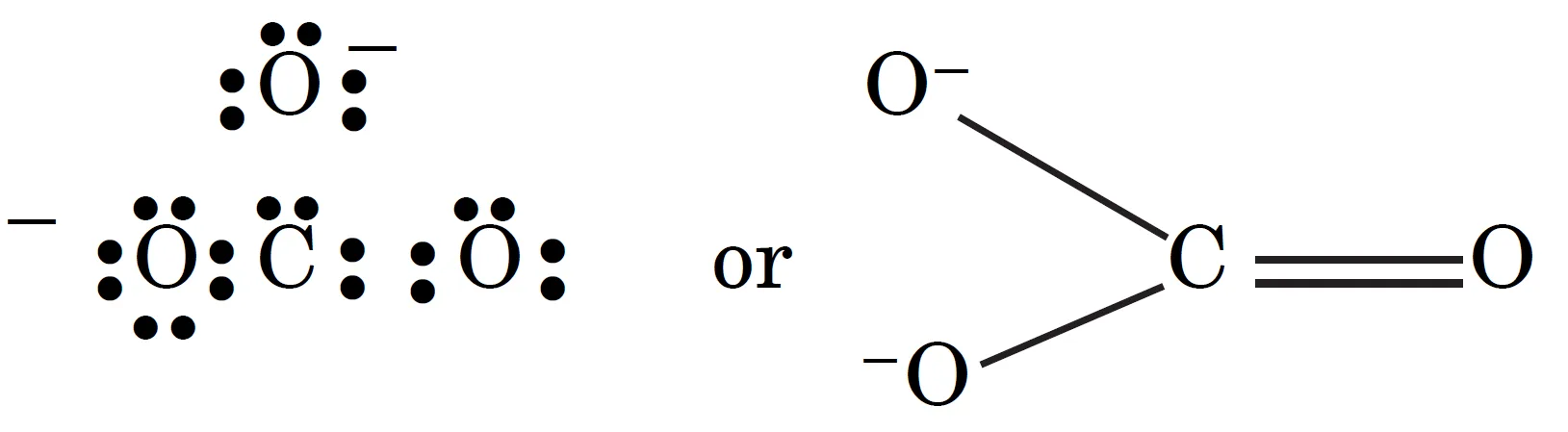
In this case, each atom has an octet of electrons. According to this structure, there are single bonds between two carbon—oxygen atoms and one double bond between carbon and oxygen atoms. Therefore, the two C—O bonds should be different than the third C=O bond.
However, experimentally, it is observed that all the three bond lengths are equal and the bonds are intermediate between single and double bonds. This means that the above Lewis structure does not account for the observed experimental facts.
To solve the problem, the CO32- ion may be represented as resonance hybrid of the following structures.
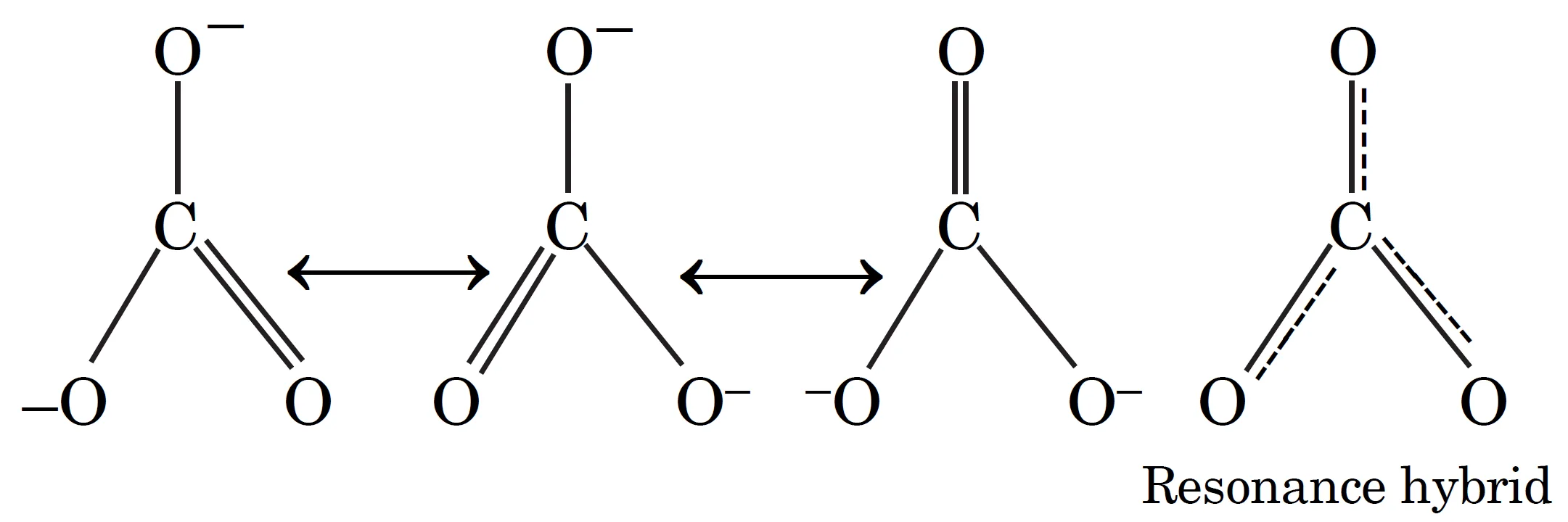
In each resonance structure:
- Each atom attains an octet.
- There are two single bonds and one double bond between carbon and oxygen.
However, experimentally, all C–O bond lengths in CO32- are identical and intermediate between a single and double bond.
This proves that none of the individual Lewis structures represents the true ion; instead, the actual structure is a resonance hybrid of all possible forms.
Key Takeaways:
- Resonance explains equal bond lengths in molecules like CO32-.
- The actual structure is a hybrid of multiple resonance forms.
- Resonance increases stability and delocalization of electrons.
Q : H3PO3 can be represented by structures 1 and II shown below. Can these two structures be taken as the canonical forms of the resonance hybrid representing H3PO3 ? If not, give reasons for the same.
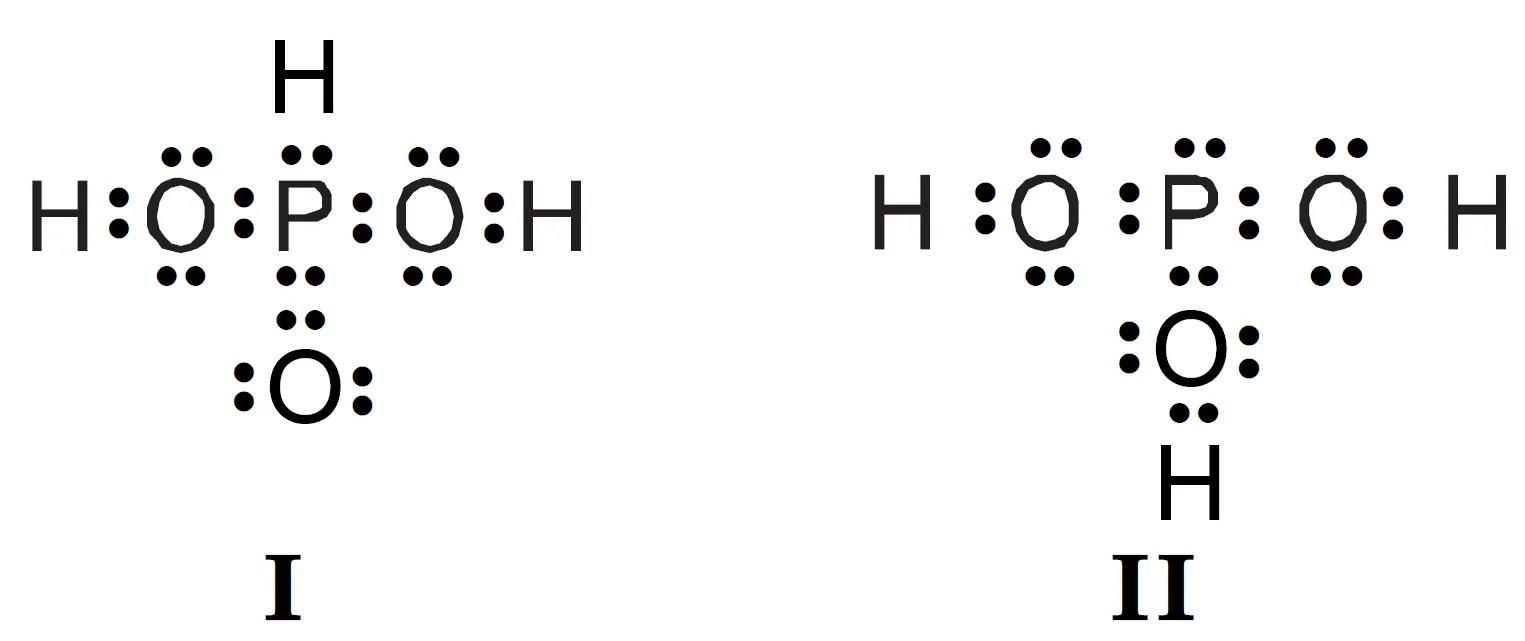
Answer:
No, the two structures of H3PO3 cannot be considered as canonical (resonance) forms because the positions of atoms change between them.
In resonance, only the positions of electrons (π-electrons or lone pairs) change — the atomic nuclei must remain fixed.
Since in H3PO3 Structures, the atomic arrangement differs, these cannot be true resonance structures.
Key Takeaways:
- Canonical forms differ only by electron positions, not by atomic positions.
- H3PO3 Structures structures are tautomers, not resonance forms.
Q : Write the Resonance Structures for SO3, NO2, and NO3–
Answer:
The resonance structures are as follows:
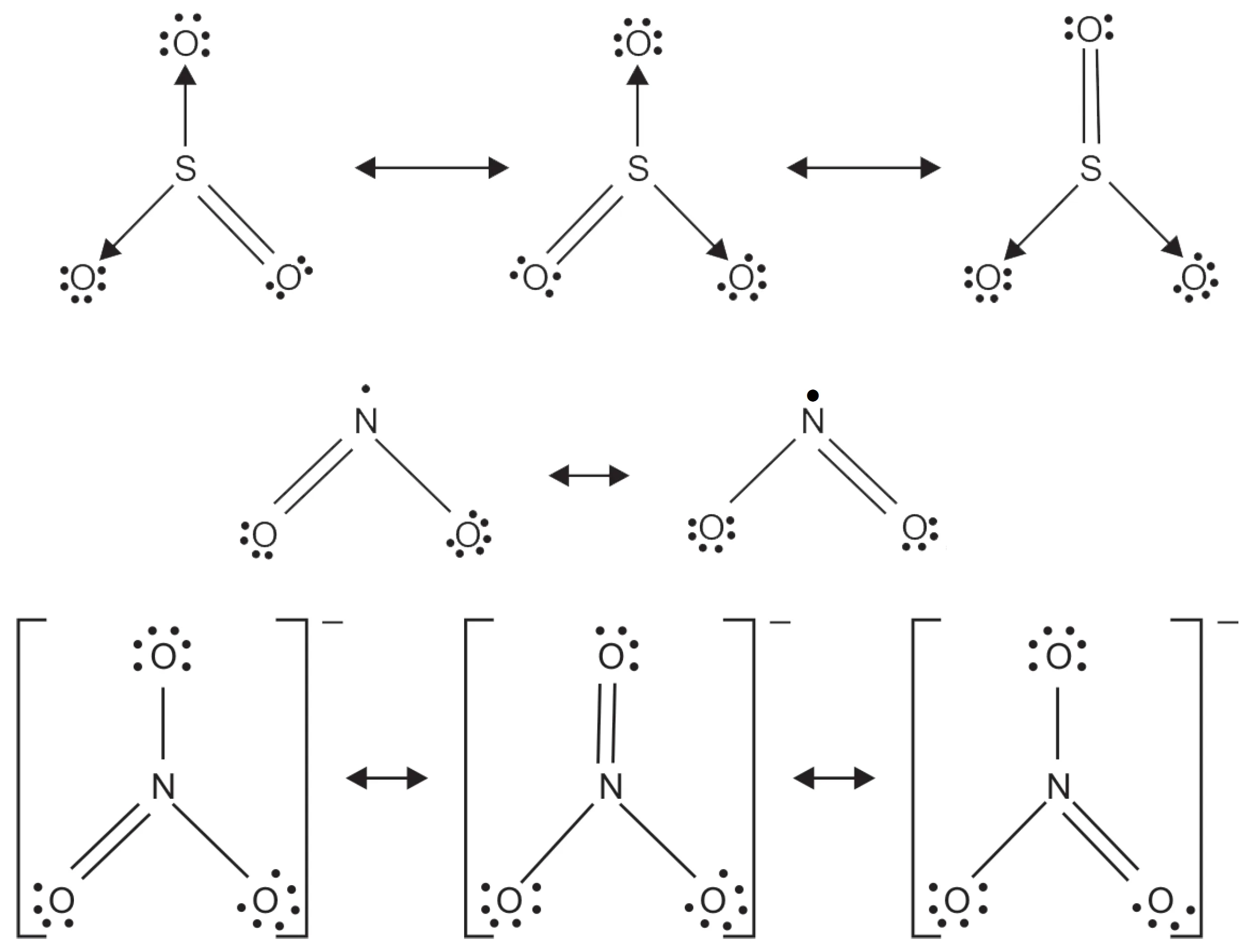
Key Takeaways:
- Each structure contributes to the resonance hybrid.
- Actual bond lengths are equal and intermediate between single and double bonds.
Q : Use Lewis Symbols to Show Electron Transfer Between the Following Atoms to form cations and anions :
(i) K and S (ii) Ca and O (ii) Al and N
Answer:
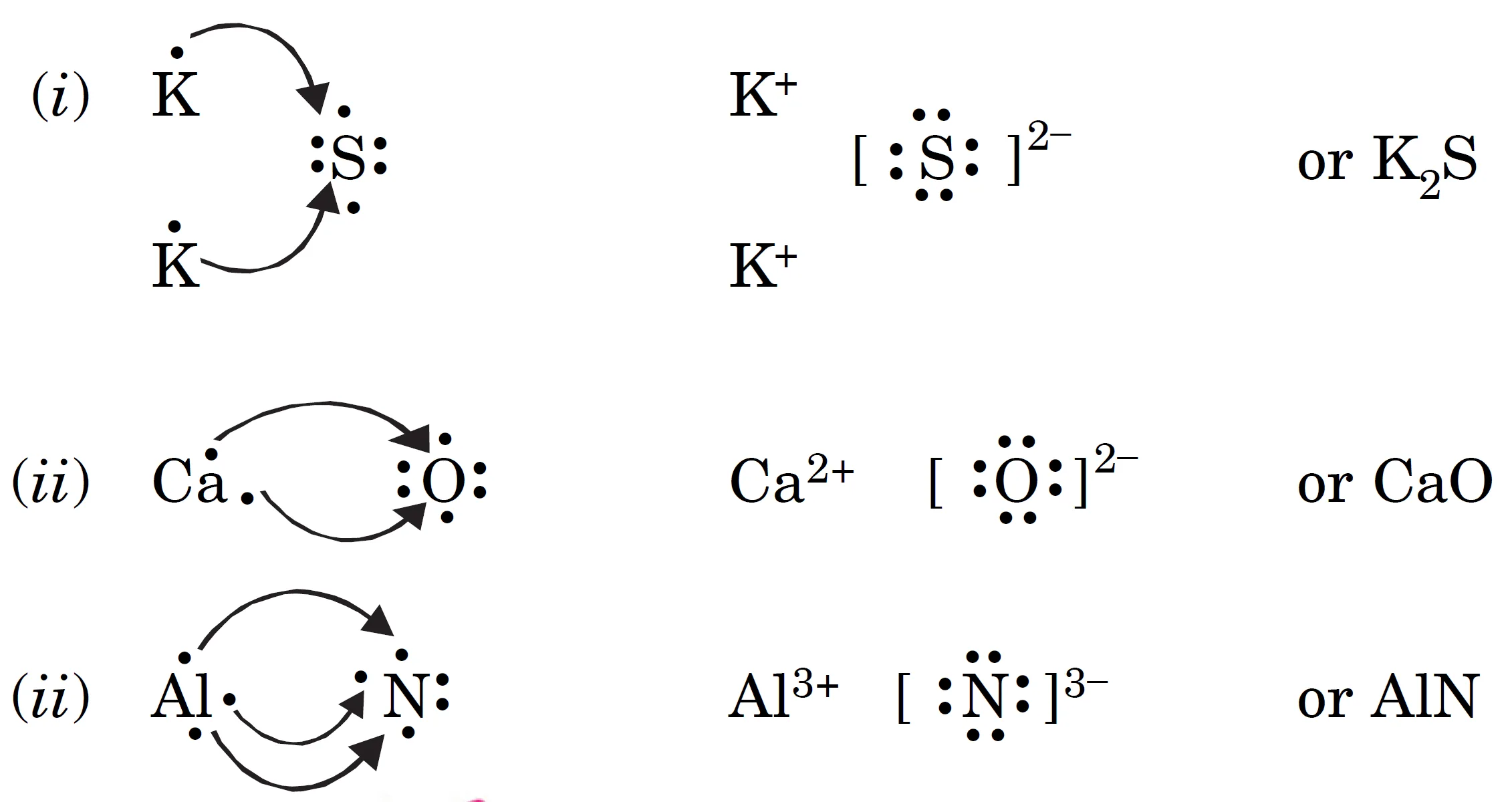
Key Takeaways:
- Ionic bonds form by complete electron transfer.
- Metals lose electrons, non-metals gain electrons.
Q : Although both CO2 and H2O are triatomic molecules, the shape of H2O molecule is bent while that of CO2 is linear. Explain this on the basis of dipole moment
Answer:
- CO2 has zero dipole moment, which means that it is linear.
The two C=O bond dipoles cancel each other due to the 180° geometry. - H2O has a net dipole moment, proving it is not linear.
It has a bent or angular shape because the O–H bond moments do not cancel out.
Key Takeaways:
- Linear molecules (e.g., CO2) → net dipole moment = 0.
- Bent molecules (e.g., H2O) → net dipole moment ≠ 0 due to lone pair–bond pair repulsion.
Q : What Are the Significance and Applications of Dipole Moment?
Answer:
Significance / Applications:
- Determines molecular polarity — nonpolar or polar.
- Indicates molecular geometry — e.g., CO2 (linear), H2O (bent).
- To distinguish between cis and trans isomers.
- To distinguish between ortho, meta and para isomers
- Used to calculate percentage ionic character of a bond.
- Helps predict physical properties like solubility and boiling point.
Key Takeaways:
- Dipole moment helps in understanding polarity, geometry, and reactivity of molecules.
Q : Define Electronegativity. What is the Difference Between Electronegativity and Electron Gain Enthalpy ?
Answer:
Although both electronegativity and electron gain enthalpy describe an atom’s ability to attract electrons, they differ in definition, measurement, and chemical context.
- Electronegativity is the tendency of an atom to attract the shared pair of electrons towards itself in a covalent bond.
- Electron gain enthalpy is the energy released when an isolated gaseous atom gains an electron to form an anion.
Difference Between Electronegativity and Electron Gain Enthalpy
| Property | Electronegativity | Electron Gain Enthalpy |
|---|---|---|
| Definition | Tendency of an atom to attract shared electrons in a covalent bond. | Energy change when an isolated gaseous atom gains an electron to form an anion. |
| Measured in | Dimensionless (Pauling scale or Mulliken scale). | kJ/mol (energy units). |
| Depends on | Bonded state of the atom in a molecule. | Isolated gaseous atom, not bonded. |
| Nature of property | Relative property, varies in compounds. | Absolute property, measured experimentally. |
| Variation across a period | Increases from left to right due to increasing nuclear charge. | Becomes more negative (more energy released) from left to right. |
| Variation down a group | Decreases down a group due to increasing atomic size. | Becomes less negative down the group due to weaker attraction for electrons. |
| Example | Fluorine has the highest electronegativity (4.0), followed by oxygen (3.5). | Chlorine releases more energy on gaining an electron than fluorine. |
| Application | Helps predict bond polarity and bond type (ionic or covalent). | Determines the ease of anion formation and reactivity of non-metals. |
Key Takeaways:
- Electronegativity → property of bonded atoms, indicates tendency to attract shared electrons.
- Electron gain enthalpy → property of isolated atoms, measures energy released during electron gain.
- Electronegativity is dimensionless, while electron gain enthalpy is measured in kJ·mol⁻¹.
Q : Explain with the help of suitable example polar covalent bond.
Answer:
When two atoms with different electronegativities share electrons unequally, the bond formed is called a polar covalent bond. Greater the difference in the electronegativities of the atoms forming the bond, greater will be the polarity of the molecule.
Example:
In H–Cl, chlorine (more electronegative) attracts the shared pair of electrons more strongly, becoming partially negative (δ-), while hydrogen becomes partially positive (δ+). For example :
Hδ+ – Clδ- , Hδ+ – Brδ- , Brδ+ – Clδ- , Oδ+ – Hδ-
Key Takeaways:
- Unequal sharing of electrons creates partial charges.
- Larger electronegativity difference → greater polarity.
Q : Arrange the Bonds in Order of Increasing Ionic Character in the molecules LiF, K2O, N2, SO2, and ClF3
Answer:
N2 < SO2 < ClF3 < K2O < LiF
Explanation:
Ionic character increases with increasing electronegativity difference between bonded atoms.
LiF shows maximum ionic character, while N2 is purely covalent.
Key Takeaways:
- Ionic character ∝ ΔElectronegativity
- N2 → least ionic (nonpolar covalent)
- LiF → most ionic.
Q : The skeletal structure of CH3COOH as shown below is correct, but some of the bonds are shown incorrectly. Write the correct Lewis structure for acetic acid ?
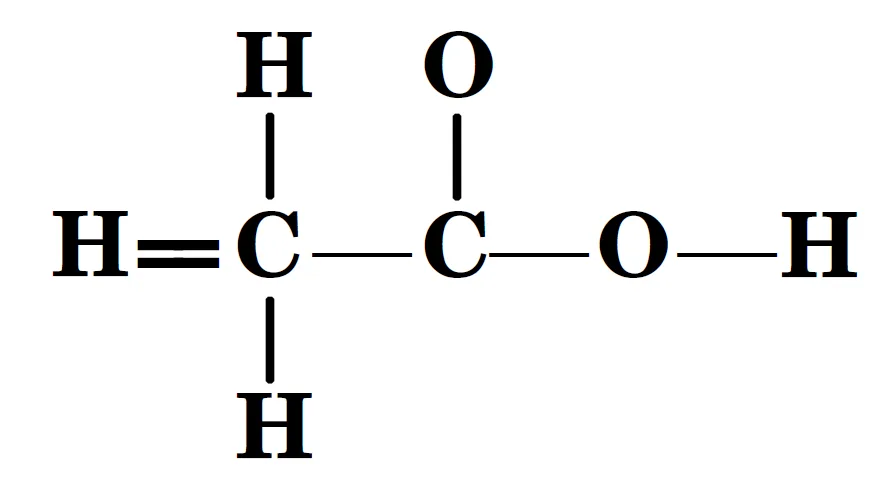
Answer:
The correct Lewis structure of acetic acid is:
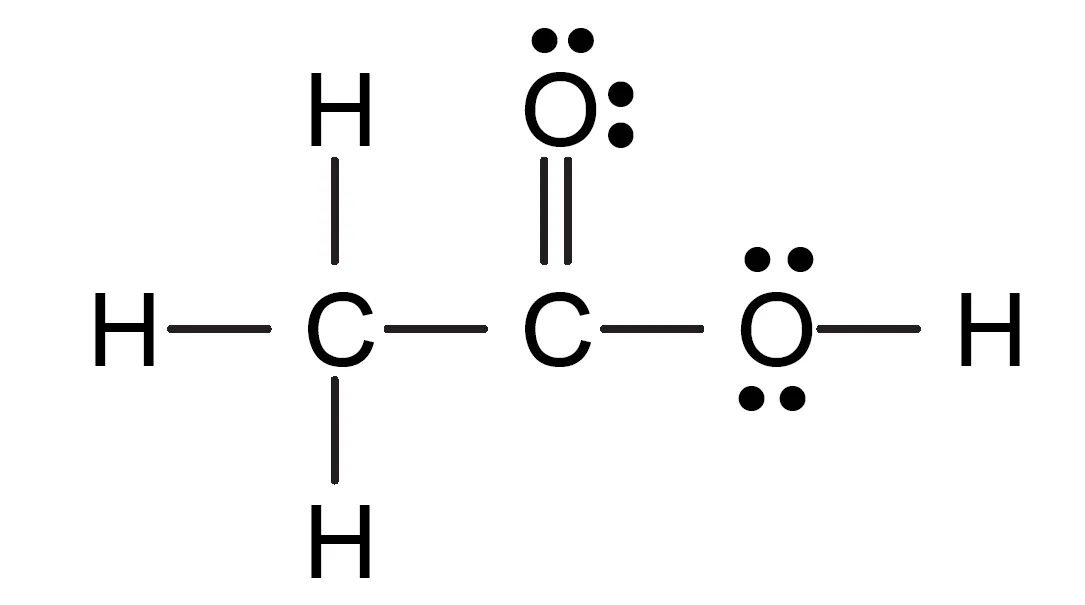
Explanation:
- One carbon forms three single bonds with hydrogen and one single bond with another carbon.
- The second carbon is double-bonded to one oxygen and single-bonded to another oxygen (which carries a hydrogen atom).
Key Takeaways:
- Acetic acid contains one C=O and one C–O–H group.
- It obeys the octet rule for all atoms.
🔍 Summary: Resonance, Dipole Moment, and Polarity in Chemical Bonding
This article explains key bonding concepts from Class 11 Chemistry NCERT, including resonance, dipole moment, electronegativity, and polarity.
- Resonance stabilizes molecules by electron delocalization.
- Dipole moment helps determine molecular shape and polarity.
- Electronegativity differences lead to polar covalent or ionic bonds.
- Understanding these helps predict bond strength, geometry, and reactivity.


