Anand Classes provides NCERT Exemplar Solutions for Class 11 Chemistry Chapter 4 – Chemical Bonding and Molecular Structure with detailed explanations, important concepts, solved MCQs, short and long answer questions, and numerical problems. These exemplar notes help students understand key topics like Lewis structures, VSEPR theory, Valence Bond Theory, Hybridisation, Molecular Orbital Theory (MOT), Bond Order, Bond Angle, Polarity, and Hydrogen Bonding. Each question is solved with step-by-step reasoning to strengthen conceptual understanding and prepare for CBSE, JEE, and NEET exams. Click the print button to download study material and notes.
Multiple-Choice Questions – MCQs (Type-I)
Q.1 Isostructural species are those which have the same shape and hybridisation. Among the given species, identify the isostructural pairs. (i) [NF3 and BF3] (ii) [BF4– and NH4+] (iii) [BCl3 and BrCl3] (iv) [NH3 and NO3–]
Solution:
Option (ii) is the answer.
(a) NF3 is pyramidal whereas BF3 is planar triangular.
(b) BF4– and NH4+ ions both are tetrahedral and sp3 hybridisation.
(c) BCl3 is triangular planar whereas BrCl3 is T shaped.
(d) NH3 is pyramidal whereas NO3– is triangular planar.
Q.2 : Polarity in a molecule and hence the dipole moment depends primarily on electronegativity of the constituent atoms and shape of a molecule. Which of Does the following have the highest dipole moment?
(i) CO2 (ii) HI (iii) H2O (iv) SO2
Solution:
Option (iii) is the answer.
CO2 molecule :
CO2 being symmetrical has zero dipole moment.
- Linear and symmetrical: The molecule’s linear shape (
O=C=O) is key to its symmetry.
- Polar bonds: Each
C=O bond is polar because oxygen is more electronegative than carbon, creating a separation of charge within each bond.
- Cancellation of dipoles: The two bond dipoles are equal in strength and point in exactly opposite directions, so they cancel each other out.
- Zero net dipole moment: Because the individual dipoles cancel completely, the molecule as a whole has no net dipole moment, making it nonpolar.
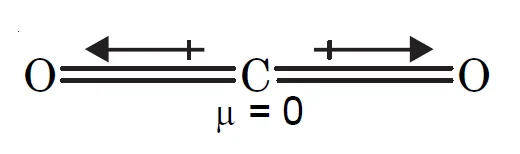
Hydrogen iodide (HI) molecule :
The hydrogen iodide (HI) molecule has a non-zero dipole moment because there is an unequal sharing of electrons between the hydrogen and iodine atoms due to a difference in their electronegativity.
- Cause: The dipole moment arises from the difference in electronegativity between hydrogen (2.2) and iodine (2.5). Since iodine is more electronegative, it attracts the shared electron pair more strongly, giving it a partial negative charge (
δ−) and leaving the hydrogen atom with a partial positive charge (
δ+).
- Magnitude: The electronegativity difference is the small, consequently, the HI bond is the less polar A common observed value for the HI dipole moment is 0.38 Debye (D).
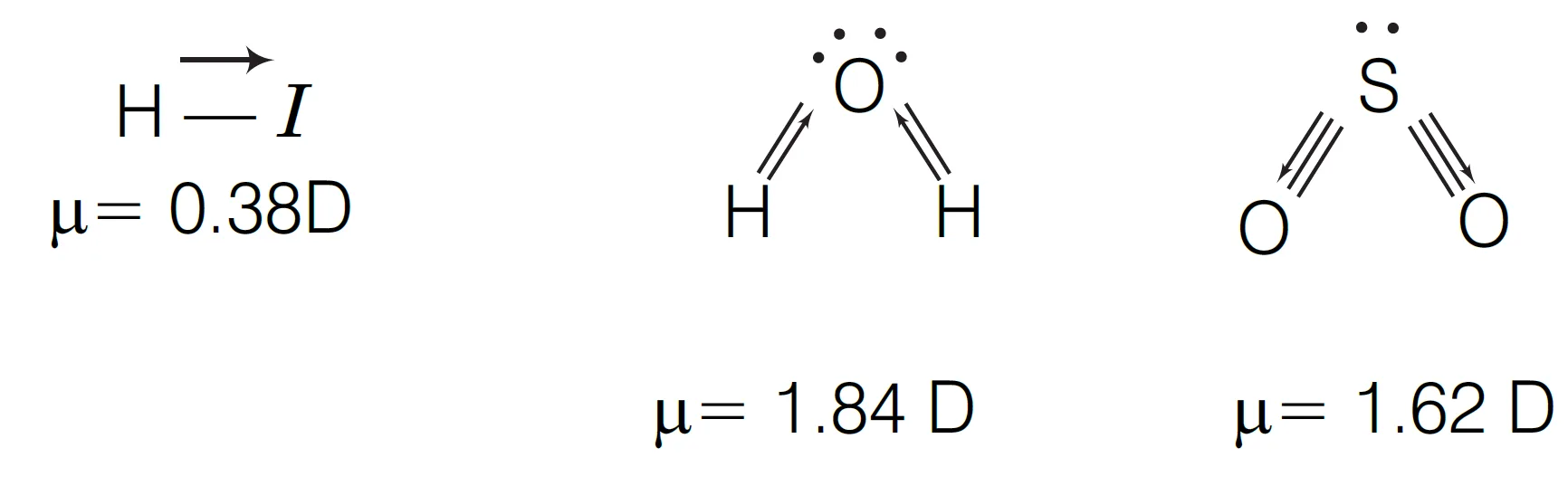
Water (H2O) and sulfur dioxide (SO2) molecules :
Water (H2O) has a higher dipole moment than sulfur dioxide (SO2) because the greater electronegativity difference between oxygen and hydrogen creates more polar bonds, leading to a larger net dipole. While both are bent molecules, the O−H bonds in water are more polar than the
S−O bonds in sulfur dioxide.
- Bond Polarity: Water has a larger electronegativity difference between oxygen (3.44) and hydrogen (2.20) compared to the difference between sulfur (2.58) and oxygen (3.44). This makes the individual
O−H bonds in water more polar than the
S−O bonds in
SO2.
- Molecular Geometry: Both molecules have a bent shape due to lone pairs on the central atom, which prevents the bond dipoles from canceling each other out. This bent geometry is crucial for both molecules to have a net dipole moment.
Q.3 : The types of hybrid orbitals of nitrogen in NO2+, NO3– and NH4+ respectively are expected to be (i) sp, sp3 and sp2 (ii) sp, sp2 and sp3 (iii) sp2, sp and sp3 (iv) sp2, sp3 and sp
Solution:
Option (ii) is the answer.
The type of hybrid orbitals of nitrogen can be decided by using VSEPR theory counting bond pairs (bp) and lone pairs (lp) in :
- NO2+ = 2 bp + 0 lp = linear => sp hybridised
- NO3– = 3 bp + 0 lp => sp2 hybridised
- NH4+ = 4 bp + 0 lp => sp3 hybridised
4. Hydrogen bonds are formed in many compounds e.g., H2O, HF, NH3. The boiling point of such compounds depends to a large extent on the strength of hydrogen bond and the number of hydrogen bonds. The correct decreasing order of the boiling points of the above compounds is :
(i) HF > H2O > NH3 (ii) H2O > HF > NH3 (iii) NH3 > HF > H2O (iv) NH3 > H2O > HF
Solution:
Option (ii) is the answer.
Why Is the Boiling Point Order H2O > HF > NH3?
1. Concept of Boiling Point
The boiling point of a substance depends mainly on the strength of intermolecular forces present between its molecules.
The stronger the intermolecular forces, the higher the boiling point.
2. Types of Intermolecular Forces
The key intermolecular forces are:
- London dispersion forces (van der Waals)
- Dipole–dipole interactions
- Hydrogen bonding
Among these, hydrogen bonding is the strongest and has the greatest effect on the boiling points of polar molecules.
3. Hydrogen Bonding in H2O , HF , NH3
Hydrogen bonding occurs when hydrogen is bonded to a highly electronegative atom (F, O, or N) and is attracted to lone pairs on nearby molecules.
Let’s analyze:
| Molecule | Electronegativity of Central Atom | No. of Hydrogen Atoms | No. of Lone Pairs on Central Atom | Type of H-bond Network | Relative H-bond Strength |
|---|---|---|---|---|---|
| NH₃ | 3.0 (N) | 3 | 1 | Limited H-bonding (each N can form 1 H-bond) | Weak |
| HF | 4.0 (F) | 1 | 3 | Linear chain H-bonding | Strong |
| H₂O | 3.5 (O) | 2 | 2 | Extensive 3D network (each O can form 4 H-bonds) | Very strong |
(a) H2O (Highest Boiling Point)
- Each water molecule forms four hydrogen bonds (two as donor, two as acceptor).
- This forms an extensive 3D network of hydrogen bonding in the liquid state.
- Hence, a large amount of energy is needed to break these bonds.
- Boiling point of H2O = 373 K
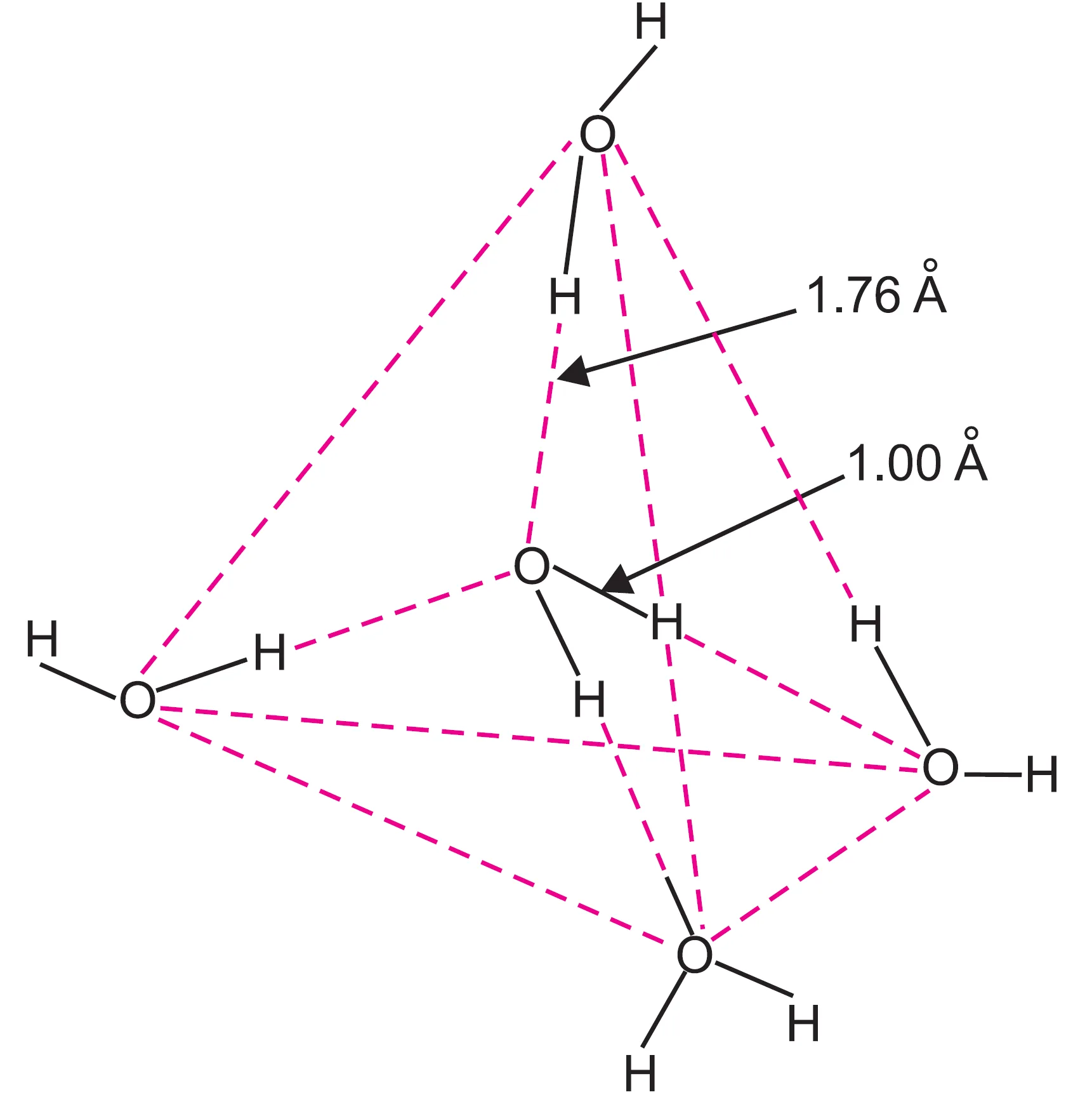
(b) HF (Intermediate Boiling Point)
- HF molecules form very strong hydrogen bonds due to the highest electronegativity of fluorine.
- However, each HF molecule can form only two hydrogen bonds (one donor, one acceptor).
- The structure is a linear chain, not a 3D network.
- Boiling point of HF = 293 K
(c) NH3 (Lowest Boiling Point)
- Although nitrogen is electronegative, each NH₃ molecule has only one lone pair and three hydrogens.
- Therefore, each molecule can form only one hydrogen bond, leading to less extensive bonding.
- Boiling point of NH3 = 239 K
Final Order of Boiling Points : H2O > HF > NH3
Key Reason : The order is due to extent of hydrogen bonding network, not just individual bond strength:
- H2O → Maximum number of hydrogen bonds (3D network)
- HF → Strong but linear hydrogen bonds (1D chain)
- NH3 → Weak and fewer hydrogen bonds
Thus extent of hydrogen bonding : H2O > HF > NH3
and Boiling point order : H2O > HF > NH3
Key Takeaways
- Boiling point depends on hydrogen bonding.
- Water forms the most extensive hydrogen-bonded network, hence has the highest boiling point.
- HF has strong but less extensive hydrogen bonds.
- NH3 shows weak and fewer hydrogen bonds.
Q.5 : In PO43- ion, the formal charge on the oxygen atom of P–O bond is
(i) + 1 (ii) – 1 (iii) – 0.75 (iv) + 0.75
Solution:
Option (ii) is the answer.
In a phosphate ion PO43-, the total charge on the ion is –3, and there are 4 oxygen atoms bonded to phosphorus.
Hence, the formal charge on each oxygen atom is:
$$
\text{Formal charge on each O atom} = \frac{\text{Total charge}}{\text{Number of O atoms}}
$$
Substituting the values:
$$
\text{Formal charge} = \frac{-3}{4} = -0.75
$$
Therefore, the formal charge on each oxygen atom in PO43- ion is –0.75.
Q.6 : In NO3– ion, the number of bond pairs and lone pairs of electrons on the nitrogen atom is
(i) 2, 2 (ii) 3, 1 (iii) 1, 3 (iv) 4, 0
Solution:
Option (iv) is the answer.
To solve this question, we must know the structure of NO3– ion.
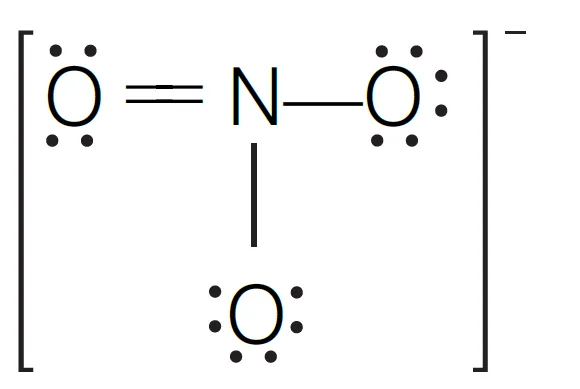
Then, count the bond pairs and lone pairs of electron on nitrogen.
- In N-atom, number of valence electrons = 5
- Due to the presence of one negative charge, number of valence electrons = 5 + 1 = 6
- One O-atom forms two bond (= bond) and two O-atom shared with two electrons of N-atom
- Thus, 3 O-atoms shared with 8 electrons of N-atom.
- Number of bond pairs (or shared pairs) = 4
- Number of lone pairs = 0
Q.7 : Which of the following species has tetrahedral geometry?
(i) BH4– (ii) NH2– (iii) CO32- (iv) H3O+
Solution:
Option (i) is the answer.
- BH4– : 4 bond pairs + 0 lone pair => sp3 hybridised = tetrahedral geometry
- NH2– : V- shape
- CO32- = triangular planar
- H3O+ : pyramidal
Q.8 : Number of π bonds and σ bonds in the following structure is–
(i) 6, 19 (ii) 4, 20 (iii) 5, 19 (iv) 5, 20
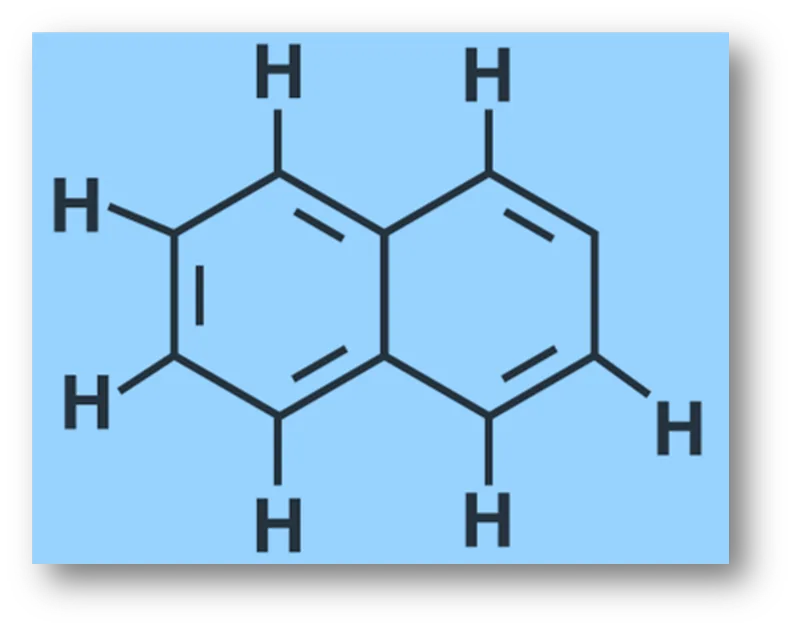
Solution:
Option (iii) is the answer.
There are 5 π-bonds and 8 C- H + 1 1C – C
σ-bonds, i.e., 19 σ-bonds are present in the above molecule.
Q.9 : Which molecule/ion out of the following does not contain unpaired electrons?
(i) N2+ (ii) O2 (iii) O22- (iv) B2
Solution:
Option (iii) is the answer.
The electronic configuration of the given molecules are :
$$
N_2^+ = (σ1s)^2(σ^*1s)^2(σ2s)^2(σ^*2s)^2(π2p_x)^2(π2p_y)^2(σ2p_z)^1
$$
N2+ has one unpaired electron.
$$
O_2 = (σ1s)^2(σ^*1s)^2(σ2s)^2(σ^*2s)^2(σ2p_z)^2(π2p_x)^2(π2p_y)^2(π^*2p_x)^1(π^*2p_y)^1
$$
O2 has two unpaired electrons
O22- : $(σ1s)^2(σ^*1s)^2(σ2s)^2(σ^*2s)^2(σ2p_z)^2(π2p_x)^2(π2p_y)^2(π^*2p_x)^2(π^*2p_y)^2
$
Thus, O22- has no unpaired electrons.
$$
B_2 = (σ1s)^2(σ^*1s)^2(σ2s)^2(σ^*2s)^2(π2p_x)^1(π2p_y)^1
$$
Thus, B2 has two unpaired electrons
Q.10 : In which of the following molecules/ions all the bonds are not equal?
(i) XeF4 (ii) BF4– (iii) C2H4 (iv) SiF4
Solution:
Option (iii) is the answer.
XeF4 : 4 bp + 2 lp => square planar => all bonds are equal
BF4– : 4 bp + 0 lp => tetrahedral (all bonds are equal)
C2H4 :
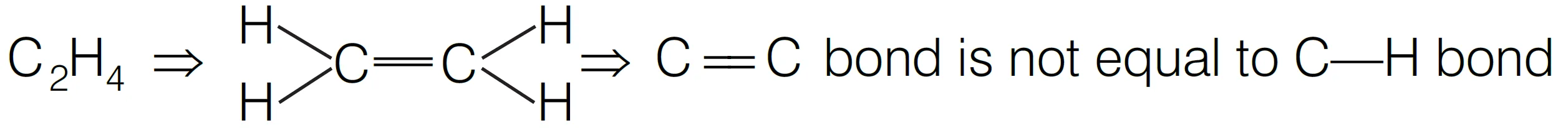
SiF4 : 4 bp + 0 lp => tetrahedral (all bonds are equal)
Thus, in C2H4 all the bonds are not equal


