Anand Classes provides a complete guide on Hybridisation – Characteristics, Conditions, and Promotion Concept, covering the fundamental principles of orbital mixing in chemical bonding. Students will learn how hybridisation explains the geometry of molecules, bond angles, and stability with the help of solved examples and diagrams. The study material also includes MCQs, Q&A, Assertion-Reason, and Case Study-based questions designed for Class 11, Class 12, JEE, and NEET exams. Click the print button to download study material and notes.
What is Hybridisation?
To explain the characteristic geometrical shapes of polyatomic molecules, Linus Pauling introduced the concept of hybridisation.
How Does Hybridisation Explain the Shape of Methane (CH4)?
To understand the concept of hybridisation, let us consider a simple molecule, methane (CH4).
Ground state electronic configuration of carbon:
$$
1s^2 ; 2s^2 ; 2p_x^1 ; 2p_y^1 ; 2p_z^0
$$
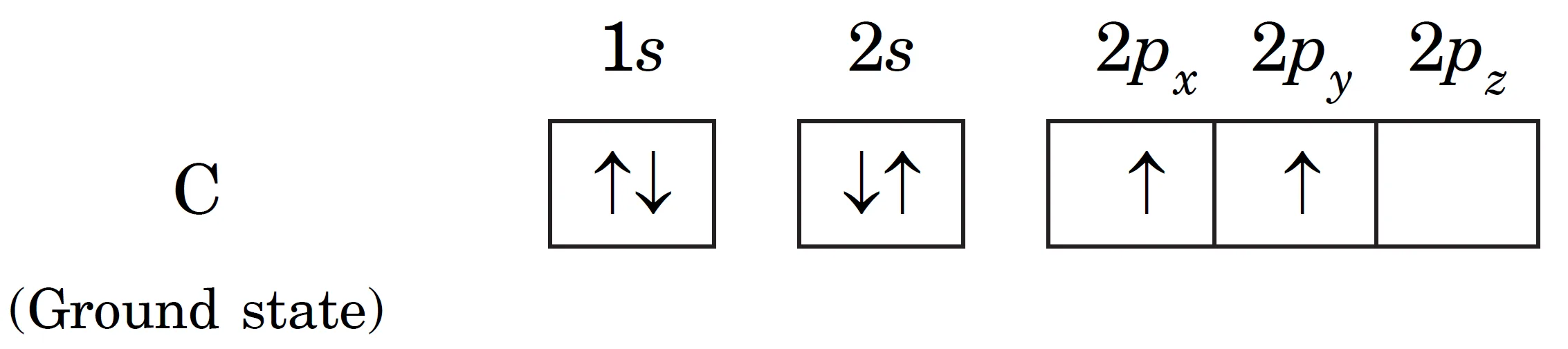
- In this state, carbon has only two half-filled orbitals in the valence shell, so it should form only two bonds (e.g., $CH_2$, $CCl_2$).
- But in practice, carbon shows valency of four, forming molecules like $CH_4$, $CCl_4$.
What is the Promotion Concept in Hybridisation?
- To explain the tetracovalency of carbon, one electron from the 2s orbital is promoted to the vacant 2pz orbital, which is in a higher energy state.
- Now, carbon has four half-filled orbitals in its valence shell, which account for the bonding capacity of four bonds of carbon.
- This state is known as excited state and the process is called promotion.
- Thus, four half filled orbitals get formed in the valence shell which account for the bonding capacity of four of carbon. The electronic configuration of carbon in the excited state is :
Excited state configuration:
$$
1s^2 ; 2s^1 ; 2p_x^1 ; 2p_y^1 ; 2p_z^1
$$
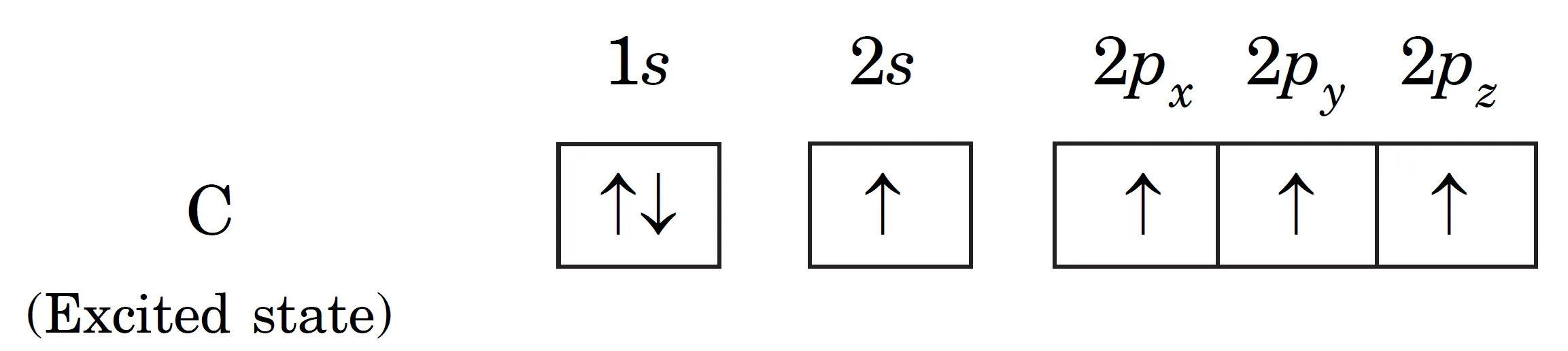
- Each of these four half filled orbitals of carbon can overlap with the 1s half filled orbital of four hydrogen atoms, forming four C–H bonds.
- However, if this overlap happened directly, the all four bonds formed by carbon would not be equivalent.
- For example, in the formation of CH4 molecule :
- one C—H bond will be formed by overlaping of 2s- orbital of C and 1s- orbital of H
- The other three C—H bonds will be formed by the overlapping of 2p- orbital of C and 1s- orbital of H.
But in CH4, all four bonds are equivalent. The equivalent character of the bonds can be explained with the help of a new concept known as hybridisation of atomic orbitals which was introduced by Pauling. This is an
important aspect of Valence Bond Theory.
From Where Promotion Energy Comes ?
The promotion of electrons from 2s to 2p orbital of higher energy level will require an input of energy. From where this energy comes? We know that the formation of covalent bonds is accompained by release of energy. Thus, when reacting atoms say H atoms approach a carbon atom to form covalent bonds, energy is released. This energy released is used to promote one electron from 2s to 2p orbital.
Promotion is not always necessary, and even filled orbitals may participate in hybridisation.
What is the Definition of Hybridisation According to Pauling?
To explain the equivalence of bonds, Pauling proposed hybridisation. According to Pauling, the atomic orbitals combine to form new set of equivalent orbitals known as hybrid orbitals. Instead of pure atomic orbitals, these hybrid orbitals are used in bond formation. This process is known as hybridisation.
Definition:
Hybridisation is the process of intermixing of atomic orbitals of slightly different energies so as to redistribute their energies to form a new set of orbitals of equivalent energy and shape.
- The new orbitals formed as a result of hybridisation are called hybrid or hybridised orbitals.
- These hybrid orbitals form stronger and equivalent bonds.
In methane, one 2s and three 2p orbitals hybridise to form four sp3 hybrid orbitals.
What are the Main Characteristics of Hybridisation?
- Number of orbitals:
Number of hybrid orbitals formed = Number of orbitals hybridised (mixed). - Energy and shape:
All hybrid orbitals are equivalent in energy and shape. - Stronger bonds:
Hybrid orbitals form more stable bonds than pure atomic orbitals. - Geometry of molecules:
Hybrid orbitals are oriented in space in specific directions → geometry of molecule depends on type of hybridisation.
What are the Conditions Required for Hybridisation?
- Only the orbitals present in the valence shell of the atom are hybridised.
- The orbitals undergoing hybridisation should have only a small difference in energy. The orbitals which differ largely in energy cannot take part in hybridisation.
- Promotion is not essential condition prior to hybridisation.
- It is not essential that only half filled orbitals participate in hybridisation. In certain cases, even filled orbitals of valence shell participate in hybridisation.
There are many types of hybridisation involving different atomic orbitals.
Short Answer Conceptual Type Questions (SAT)
Q1. What problem arises with the ground state electronic configuration of carbon in explaining its tetravalency?
Answer: In the ground state, carbon has only two half-filled orbitals ($2p_x^1, 2p_y^1$). Therefore, it should form only two bonds. But experimentally, carbon forms four bonds (e.g., in $CH_4$).
Q2. What is the promotion concept in carbon hybridisation?
Answer: One electron from the $2s$ orbital is promoted to the vacant $2p_z$ orbital, giving four half-filled orbitals. This explains the tetravalency of carbon.
Q3. From where does the promotion energy in hybridisation come?
Answer: The promotion of an electron from $2s$ to $2p$ requires energy, which is supplied by the bond formation energy released when hydrogen atoms overlap with carbon.
Q4. State Pauling’s definition of hybridisation.
Answer: Hybridisation is the process of intermixing of atomic orbitals of slightly different energies to form a new set of orbitals of equivalent energy and shape.
Q5. Why are all C–H bonds in methane equivalent?
Answer: In methane, $2s$ and $2p$ orbitals hybridise to form four $sp^3$ orbitals, which are identical and form four equivalent bonds with hydrogen.
Multiple Choice Questions (MCQs)
Q1. The ground state electronic configuration of carbon is:
(a) $1s^2 ; 2s^2 ; 2p^2$
(b) $1s^2 ; 2s^1 ; 2p^3$
(c) $1s^2 ; 2s^2 ; 2p_x^1 ; 2p_y^1 ; 2p_z^0$
(d) $1s^2 ; 2s^2 ; 2p_x^2 ; 2p_y^0 ; 2p_z^0$
Answer: (c) $1s^2 ; 2s^2 ; 2p_x^1 ; 2p_y^1 ; 2p_z^0$
Explanation: In the ground state, carbon has only two half-filled $p$ orbitals.
Q2. Which orbitals hybridise in methane to form four equivalent bonds?
(a) One $2s$ and three $2p$ orbitals
(b) Two $s$ and two $p$ orbitals
(c) Four $p$ orbitals
(d) One $s$ and one $p$ orbital
Answer: (a) One $2s$ and three $2p$ orbitals
Explanation: $2s, 2p_x, 2p_y, 2p_z$ hybridise to form four $sp^3$ orbitals.
Q3. Which statement about hybrid orbitals is correct?
(a) Hybrid orbitals have different energies.
(b) Hybrid orbitals are weaker than pure orbitals.
(c) Hybrid orbitals are equivalent in shape and energy.
(d) Hybridisation always requires promotion.
Answer: (c) Hybrid orbitals are equivalent in shape and energy.
Assertion-Reason Type Questions
Q1.
Assertion (A): All four bonds in $CH_4$ are equivalent.
Reason (R): Hybridisation forms four identical $sp^3$ orbitals which overlap with hydrogen’s $1s$ orbitals.
Options:
(a) Both A and R are true, and R is the correct explanation of A
(b) Both A and R are true, but R is not the correct explanation of A
(c) A is true, R is false
(d) A is false, R is true
Answer: (a) Both A and R are true, and R is the correct explanation of A
Q2.
Assertion (A): Promotion of electrons is always necessary before hybridisation.
Reason (R): Hybridisation can only occur with half-filled orbitals.
Answer: Both A and R are false.
Explanation: Promotion is not always necessary, and even filled orbitals may participate in hybridisation.
Case Study
Passage:
Carbon in its ground state should form only two bonds because it has two half-filled $2p$ orbitals. However, in practice it forms four bonds, e.g., in methane ($CH_4$). To explain this, one electron from the $2s$ orbital is promoted to the $2p_z$ orbital, creating four half-filled orbitals. These orbitals undergo $sp^3$ hybridisation to form four equivalent orbitals, each overlapping with hydrogen’s $1s$ orbital. Thus, methane forms four equivalent C–H bonds.
Q1. What is the excited state configuration of carbon in methane?
Answer: $1s^2 ; 2s^1 ; 2p_x^1 ; 2p_y^1 ; 2p_z^1$
Q2. What type of hybridisation occurs in methane?
Answer: $sp^3$ hybridisation
Q3. Why are all C–H bonds in methane identical?
Answer: Because $sp^3$ hybrid orbitals are equivalent in energy and shape.
Q4. From where does the energy for promotion of electron come?
Answer: From the bond formation energy released when carbon bonds with hydrogen.


