Anand Classes provides complete NCERT Solutions for Class 11 Chemistry — Chemical Bonding and Molecular Structure (Questions Q4.21, 4.22, 4.23, 4.24, 4.25, 4.26, 4.27, 4.28, 4.29, 4.30) with clear, step-by-step explanations, diagrams, and concept notes. These solutions cover important topics such as VSEPR, hybridisation, molecular orbital theory, bond order, polarity, and stability, and are prepared to match CBSE exam style while helping JEE/NEET aspirants strengthen core concepts. Click the print button to download study material and notes.
Q : Apart from tetrahedral geometry, another possible geometry for CH4 is square planar with the four H atoms at the corners of the square and the C atom at its centre. Explain why CH4 is not square planar ?
Answer:
In a square planar geometry, the bond angle between the substituents is 90°, while in a tetrahedral geometry, the bond angle is 109.5°.
Because the bond angle in the square planar structure is smaller, the repulsive forces between electron pairs increase, leading to greater instability. Therefore, CH4 adopts a tetrahedral geometry rather than a square planar one.
Key Takeaways:
- Square planar angle (90°) < Tetrahedral angle (109.5°).
- Higher electron pair repulsion makes the square planar form less stable.
- CH4 is therefore tetrahedral in shape.
Q : Explain Why BeH2 molecule Has a Zero Dipole Moment although Be–H Bonds Are Polar?
Answer:
BeH2 is a linear molecule. Although each Be–H bond is polar, the individual dipole moments act in opposite directions and cancel each other. Hence, the resultant dipole moment of BeH2 is zero.
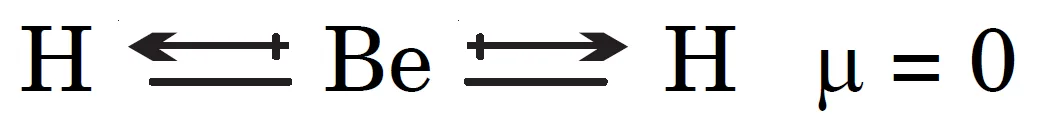
Key Takeaways:
- BeH₂ has a linear geometry.
- Bond dipoles are equal and opposite, cancelling out.
- Hence, BeH₂ has no net dipole moment.
Q : Which out of NH3 and NF3 has higher dipole moment and why ?
Answer:
NH3 has a higher dipole moment than NF3. Both molecules are pyramidal with one lone pair on the nitrogen atom.
Although fluorine is more electronegative than hydrogen, the orientation of the lone pair relative to the bond dipoles determines the net moment.
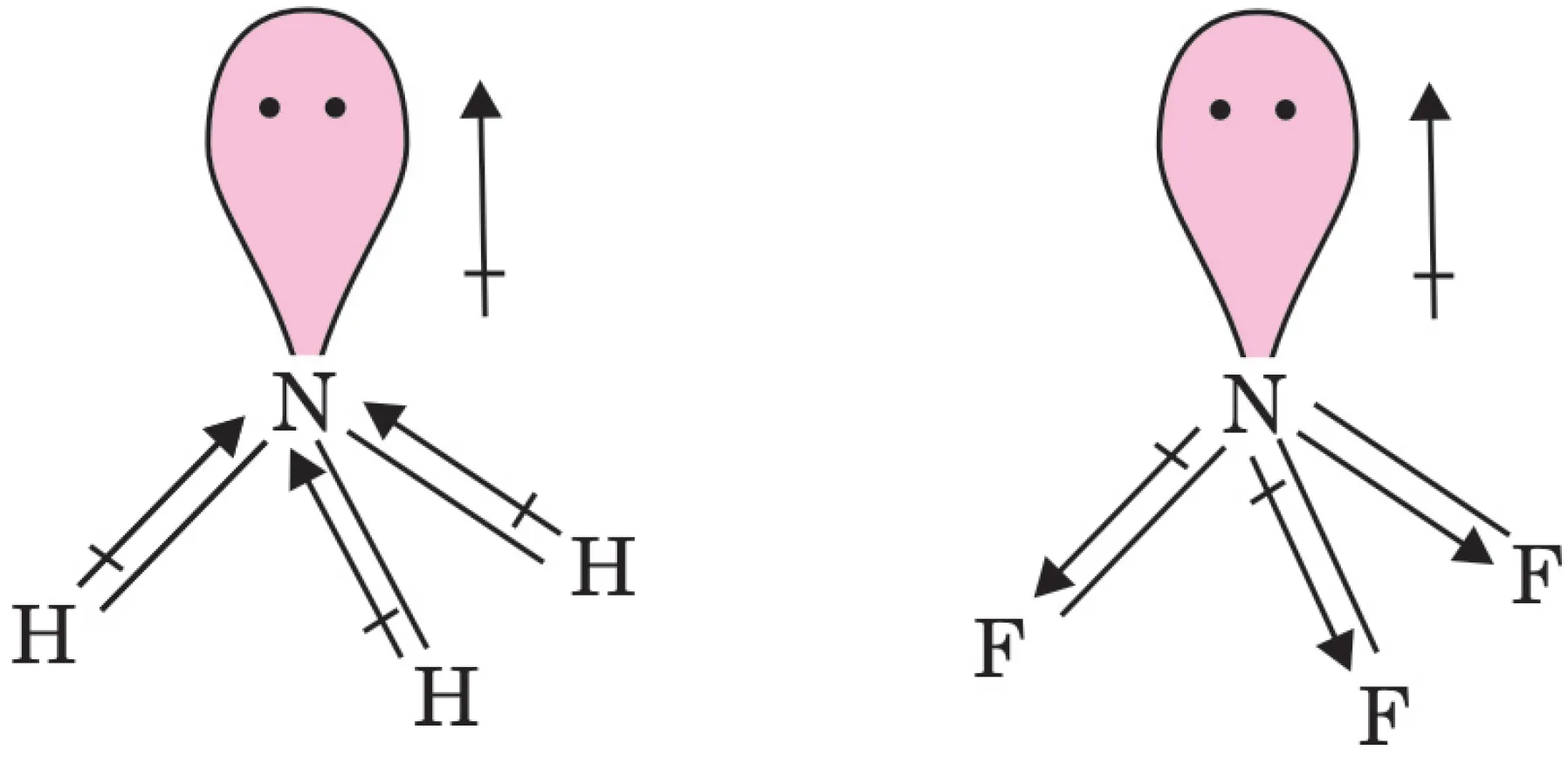
- In NH3, the lone pair dipole is in the same direction as the resultant dipole of the three N–H bonds.
- In NF3, the lone pair dipole is in the opposite direction to the resultant dipole of the three N–F bonds.
Therefore, the lone pair dipole in NH3 adds to the resultant dipole moment, while in NF3 it subtracts from it, making the overall dipole moment smaller.
Key Takeaways:
- Both NH3 and NF3 are pyramidal.
- Direction of lone pair moment decides the resultant dipole.
- NH3 → higher dipole moment (1.47 D).
- NF3 → lower dipole moment (0.24 D).
Q : What Is Meant by Hybridisation of Atomic Orbitals? Describe Shapes of sp, sp2, sp3 Hybrid Orbitals.
Answer:
Hybridization is defined as the mixing of the atomic orbitals belonging to the same atom but having slightly different energies so that a redistribution of energy takes place between them resulting in the formation of new orbitals of equal energies and identical shapes. The new orbitals thus formed are known as hybrid orbitals.
- sp hybridisation : when one s and one p orbital belonging to the same main shell of an atom mix together to form two new equivalent orbitals type of hybridisation is called sp hybridisation
- Linear shape, bond angle = 180°.
- sp2 hybridisation: when one s and two p orbitals of the same shell of an atom mix to form three new equivalent orbitals, the type of hybridization is called sp2 hybrid orbitals.
- Trigonal planar shape, bond angle = 120°.
- sp3 hybridisation: when one s and three p orbitals of the same shell of an atom mix to form four new equivalent orbitals, the type of hybridization is called sp3 hybrid orbitals.
- Tetrahedral shape, bond angle = 109.5°.
Key Takeaways:
- Hybridisation explains molecular geometry.
- The type of hybridisation depends on the number of sigma bonds and lone pairs.
- sp → Linear, sp2 → Trigonal planar, sp3 → Tetrahedral.
Q : Describe the change in hybridisation (if any) of the Al atom in the following reaction : AlCl3 + Cl– → AlCl4– ?
Answer:
Electronic Configuration of 13Al = 1s2 2s2 2p6 3s2 3p1 (Ground state) or 1s2 2s2 2p6 3s1 3px1 3py1 (Excited state). It has three unpaired electrons. It forms three covalent bonds with 3 unpaired electrons from 3 chlorine atoms. Hence, it undergoes, sp2 hybridization to give it planar triangular structure.
To form AlCl4–, the empty 3pz orbital is also involved so that hybridization is sp3 and the shape is tetrahedral.
In AlCl3 aluminium uses sp2 hybridisation. When it reacts with a chloride ion (Cl–), the coordination number increases from 3 to 4, and hybridisation changes to sp3.
$$
\text{AlCl}_3 , (\text{sp}^2) + \text{Cl}^- \rightarrow \text{AlCl}_4^- , (\text{sp}^3)
$$
Key Takeaways:
- Al in AlCl3 : sp2 hybridised.
- Al in AlCl4– : sp3 hybridised.
- Change occurs due to coordination number increase from 3 to 4.
Q : Is There Any Change in Hybridisation of B and N atoms as a result of the following reaction ?
BF3 + NH3 → F3B·NH3?
Answer:
Yes, B in BF3 (which is sp2 hybridised) accepts a lone pair from N in NH3 (which is sp3 hybridised) to form a coordinate bond, resulting in sp3 hybridisation for both atoms in F3B·NH3.
Key Takeaways:
- B (BF3): sp2 → sp3
- N (NH3): remains sp3
- Formation of coordinate bond changes geometry.
Q : Draw diagrams showing the formation of a double bond and a triple bond between carbon atoms in C2H4 and C2H2 molecules.
Answer:
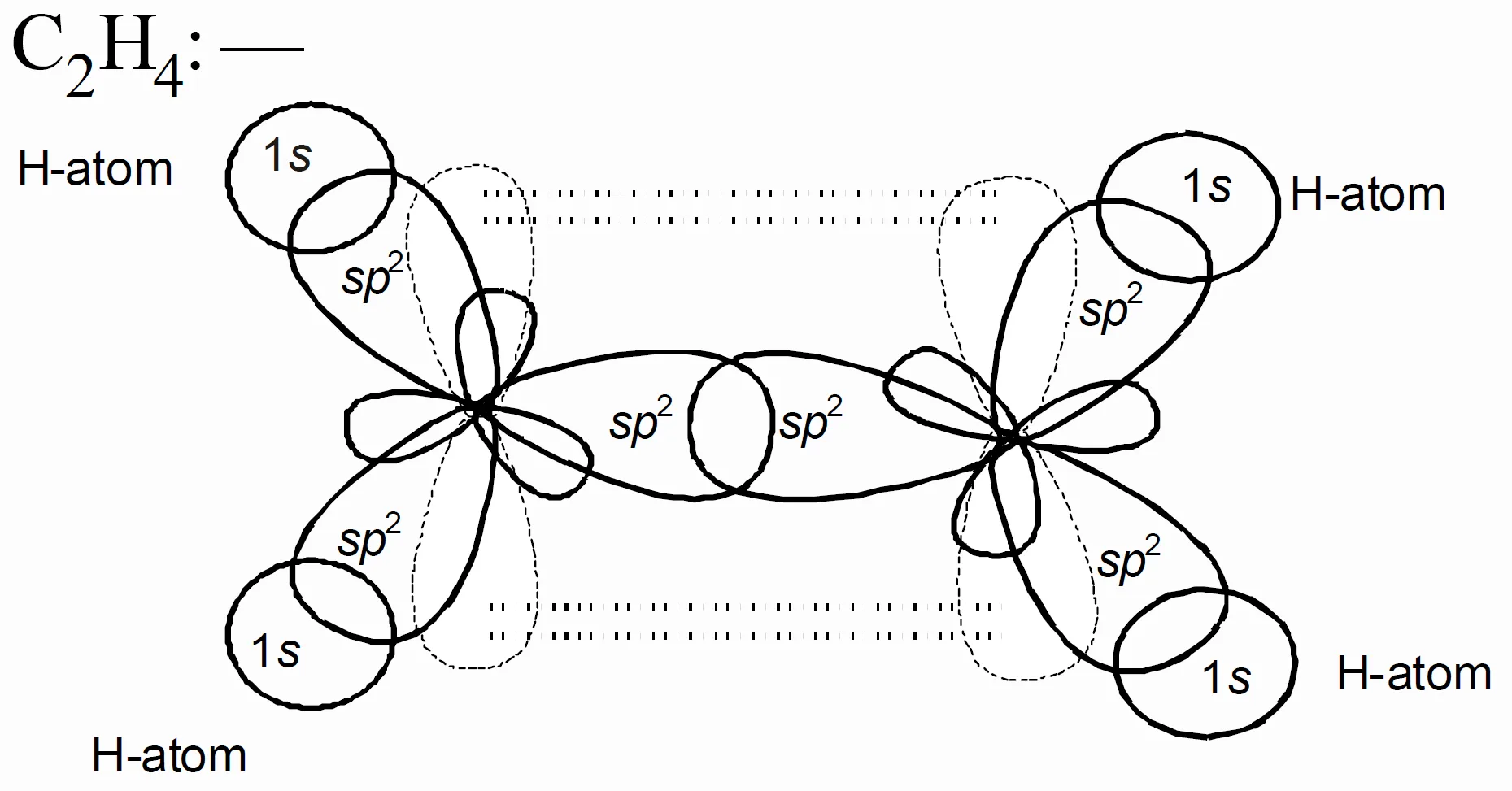
- In C2H4 (ethylene):
Each carbon is sp2 hybridised. Two sp2 orbitals form sigma bonds (σ), and the unhybridised p orbitals overlap sideways to form one π bond (π). Hence, C=C has one σ bond and one π bond.
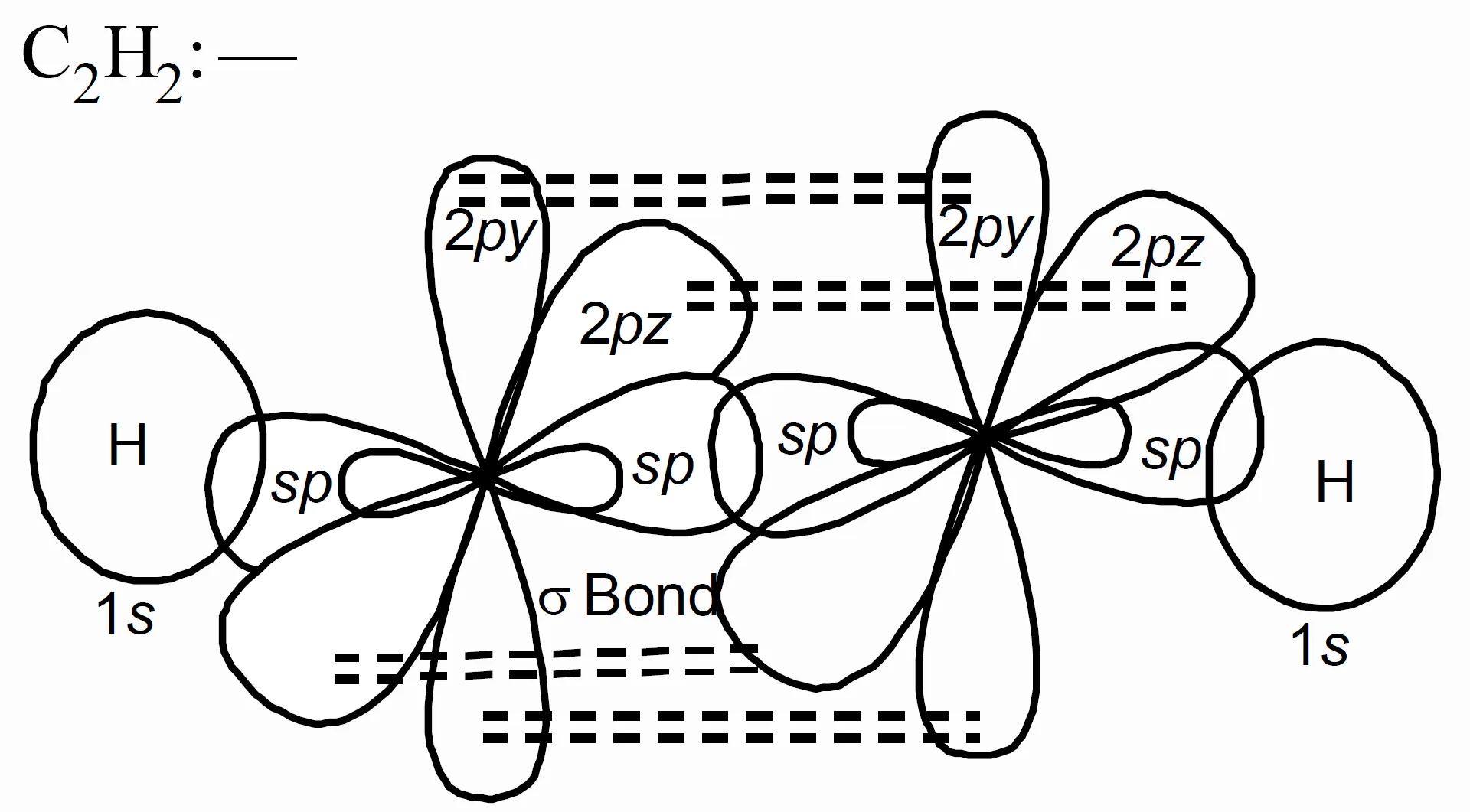
- In C2H2 (acetylene):
Each carbon is sp hybridised. One sp orbital from each carbon forms a σ bond, and the two unhybridised p orbitals form two π bonds. Hence, C≡C has one σ bond and two π bonds.
Key Takeaways:
- C2H4 (ethylene): double bond = 1 σ + 1 π.
- C2H2 (acetylene): triple bond = 1 σ + 2 π.
- σ bonds from head-on overlap, π bonds from sidewise overlap.
Q : What Is the Total Number of Sigma and Pi Bonds in following molecules (a) C2H2 and C2H4 ?
Answer:
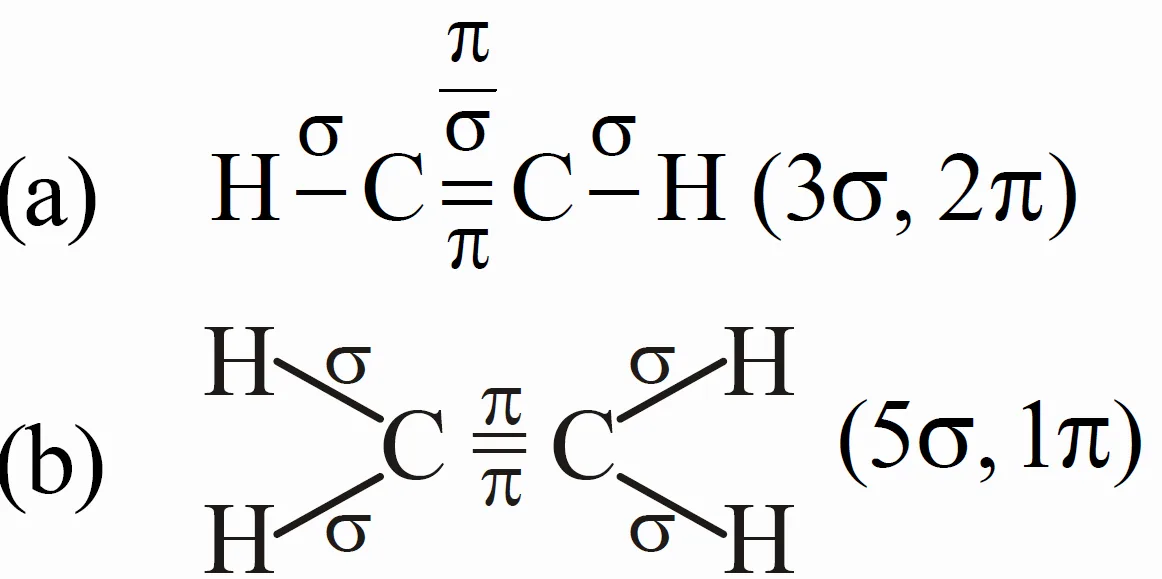
Key Takeaways:
- C2H2 : 3 σ bonds, 2 π bonds.
- C2H4 : 5 σ bonds, 1 π bond.
Q : Considering x-axis as the internuclear axis which out of the following will not form a sigma bond and why ?
(a) 1s and 1s (b) 1s and 2px (c) 2py and 2py (d) 1s and 2s.
Answer:
Taking the x-axis as the internuclear axis:
(a) 1s and 1s → σ bond formed
(b) 1s and 2px → σ bond formed
(c) 2py and 2py → No σ bond formed, forms π bond instead
(d) 1s and 2s → σ bond formed
Hence, option (c) does not form a σ bond because the sidewise overlap of 2py orbitals forms a π bond.
Key Takeaways:
- σ bonds form by end-to-end overlap along internuclear axis.
- π bonds form by sidewise overlap.
- 2py and 2py cannot form a σ bond along x-axis.
Q : Which hybrid orbitals are used by carbon atoms in the following molecules ?
(i) CH3–CH3 (ii) CH3–CH = CH2
(iii) CH3–CHO (iv) CH3COOH (v) CH3–CH2–OH
Answer:
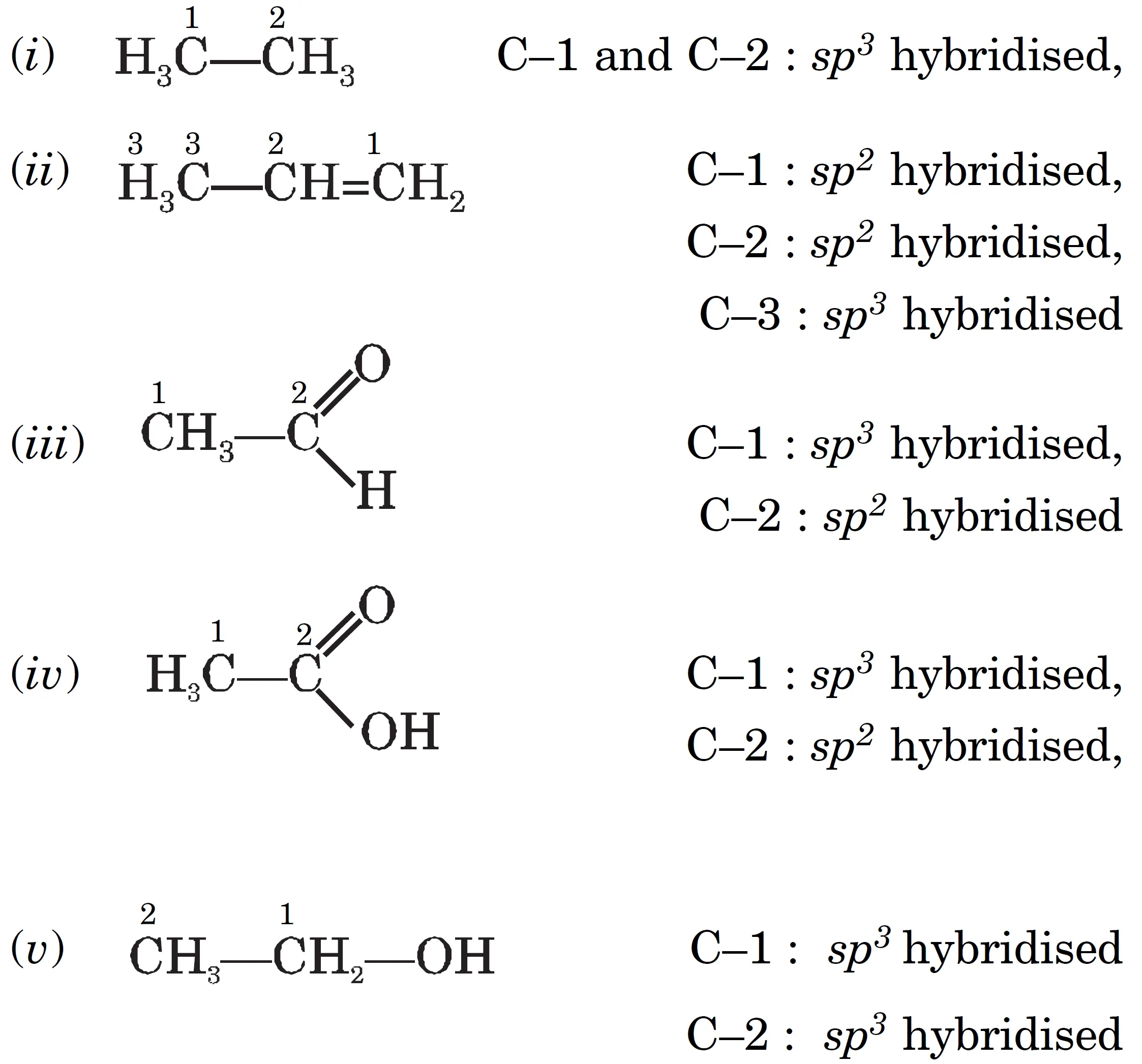
Key Takeaways:
- Single bonds: sp3
- Double bonds: sp2
- Triple bonds: sp
🔍 Summary: Understanding Molecular Geometry and Bonding Concepts
This post explains key chemical bonding concepts from Class 11 Chemistry (NCERT) including:
- Why CH₄ is tetrahedral, not square planar
- Dipole moments in polar molecules like BeH₂, NH₃, NF₃
- Hybridisation (sp, sp², sp³) and how it affects bond angles and molecular geometry
- Sigma and Pi bond formation in C₂H₂ and C₂H₄
- Changes in hybridisation during reactions like AlCl₃ + Cl⁻ → AlCl₄⁻
These fundamental concepts form the core of chemical bonding theory and are essential for understanding molecular shapes, polarity, and bond formation mechanisms in physical and inorganic chemistry.


