In daily life, one encounters many Pharmaceutical drugs, and the human’s basic needs have an addition of medicine. The word drug was derived from the French word ‘drogue’ meaning a dry herb. In this topic, let’s understand what is a drug in the first place and its analgesic effects.
Table of Contents
What is Drug ?
A drug is defined as a substance that is used for cure, relief, prevention, diagnosis of a disease. An ideal drug is the one that has the least side effects and should not disturb or interrupt the important function of the human body but destroy the disease-causing germ. Drugs need not be consumed orally, it can also be used by injecting them into the body.
The therapeutic action of different classes of drugs
- Classification of drugs depending on their medicinal uses: Most of the drugs are consumed for daily problems that occur in our body for example we use antacids for acidity problems in the stomach, antibiotics, and antiseptics to kill the bacterial growth on or in the body and tranquilizers that affect the central nervous system. Don’t worry every drug mentioned in this topic will be discussed in detail with its chemical properties.
- Antacids: Antacids are a base that neutralizes excess acid in the stomach. Inside the stomach, the body uses Hydrochloric acid for the process of digestion of food, sometimes, it is secreted in excess and causes acidity. In antacids, mostly it’s Sodium bicarbonate and metal hydroxides of magnesium and aluminum are used in antacids. Antacids are used to control the issue, they can’t cure the acidity. Some problems caused due to excess use of Antacids are diarrhoea and kidney disease example, Pepto-Bismol.
- Antibiotics: Antibiotics are drugs derived from plants or micro-organisms and are used to kill or prevent the growth of other micro-organisms. Antibiotics work in a very precise way. A certain antibiotic is only effective against a specific type of micro-organism. The range of organisms sensitive to an antibiotic’s inhibition is referred to as the antibiotic’s spectrum. Paul Ehrlich, a German bacteriologist, developed arsenic-containing drugs to cure syphilis. Paul Ehrlich won the Nobel Prize in 1908 for inventing arsphenamine (salvarsan), a substance that kills syphilis germs. He also developed azodyes with a -N = N- linkage, comparable to the -As = As- linkage found in salvarsan, and antibiotic prontosil example Penicillin.
- Antiseptics: The drugs which are used outside the body on the skin are called antiseptics. They are used to kill and stop the growth in a wound. The most common antiseptic which you may know is Dettol which is a mixture of terpineol and chloroxylenol example Chlorhexidine.
- Disinfectants: It has a similar function as Antiseptic but is used in non-living things example Chlorine and chlorine compounds.
- Classification based on the action of drugs based on the particular biochemical process: These drugs are disease dependent and affect the CNS or PNS accordingly. Examples are local anesthetic agents, painkillers, etc.
- Classification based on chemical structure: Alcohols, ketones, hydrocarbons, esters, amides, lactones, phenols, and other drugs are divided into many groups. Compounds with comparable chemical structures are expected to have similar chemical characteristics, yet biological qualities are not observed to be similar. Amino alcohols, for example, do not all have the same biological function. As a result, categorization based on drug effects is more precise.
- Classification based on a misconception of the public: The classification is based on the effect of pharmaceuticals such as cough syrups, laxatives, and home remedies, as well as analgesics, ointments, and injections. However, with an understanding of the characteristics of chemistry and the biological action of medications, this form of classification is scientifically inappropriate. Here, let’s look at drug-target interaction categorization based on molecular targets.
The action of drugs on target
- Enzyme as a drug’s target: Enzymes in their active sites keep the substrate in a suitable position for the successful assault of reagent in biochemical processes, similar to a lock and key. Enzymes hold substrate molecules together in a variety of methods, including covalent bonds, hydrogen bonds, van der Waal’s forces, and so on. Enzymes supply the required functional group that attacks the substrate, allowing chemical reactions to take place.
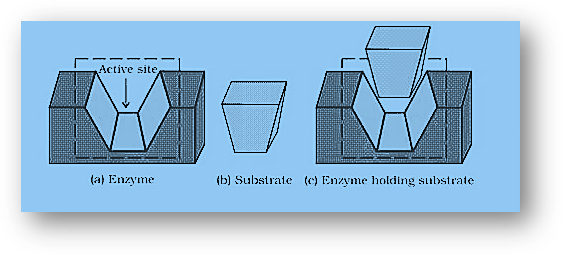
- The action of the drug in general: To connect to the active site of enzymes, medicines, and substrates compete with one another. Competitive inhibition is the term given to these medications. The medication binds to the enzyme and stops it from binding to the substrate. As a result, the medication inhibits enzyme catalysis. Some medications bind to enzymes at distinct places than the substrate and do not compete with it (allosteric sites). The structure of the enzyme changes as a result of contact at a point other than the active site, making it harder for the substrate to recognize the active site. The drug’s binding to the enzyme might be exceedingly strong at times. In such circumstances, the body produces new enzymes while the one that is inhibited by the medication degenerates.
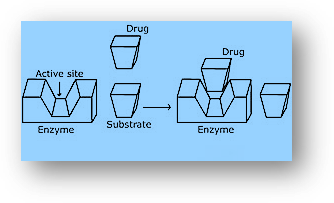
- Receptors act as a drug target: To connect to the active site of enzymes, medicines, and substrates compete with one another. Competitive inhibition is the term given to these medications. The medication binds to the enzyme and stops it from binding to the substrate. As a result, the medication inhibits enzyme catalysis. Some medications bind to enzymes at distinct places than the substrate and do not compete with it (allosteric sites). The structure of the enzyme changes as a result of contact at a point other than the active site, making it harder for the substrate to recognize the active site. The drug’s binding to the enzyme might be exceedingly strong at times. In such circumstances, the body produces new enzymes while the one that is inhibited by the medication degenerates.
Other classifications of drugs
These classified drugs below will explain some of the most consumed drugs and how they work,
- Analgesics: Drugs that relieve the pain by acting on CNS without loss of consciousness or without much disturbing the nervous system are called analgesics. There are two categories in which they have been divided,
- Narcotic Analgesics: These drugs, such as morphine, codeine, and heroin, cause central nervous system depression and provide immediate pain relief. These chemicals are used to alleviate pain caused by bone fractures, post-operative pain, and burns, among other things. The main disadvantage of these drugs is that they cause addiction and can cause effects like vomiting, mental confusion, etc. for example oxycodone and aspirin.
- Non-Narcotic Analgesics: When these medications are used, they do not cause any major central nervous system depression. They have anti-inflammatory and antipyretic properties (reduce fever). Inflammation is a set of changes in tissue that results in skin redness, tissue granulation, and other symptoms. Analgesics lower inflammation by acting as anti-inflammatory agents. Aspirin is a widely used analgesic, anti-inflammatory, and antipyretic medication. for example, acetaminophen.
- Tranquilizers: The word tranquilizer comes from the Latin word tranquillus, which means “calm.” Tranquilizers are pharmacological chemicals that are used to ease or lessen tension and anxiety, resulting in tranquility. These medications interfere with the transmission of messages from nerves to receptors, for example. Noradrenaline, a neurotransmitter, is vital in affecting a person’s mood.
- Antimicrobials: The drugs used to kill or stop the growth of these micro-organisms are called antimicrobials. These are Antibiotics, antiseptics, and disinfectants which we have already studied above.
- Antifertility Drugs: These drugs are used to control the population by family planning which can be useful because the increase in population is causing a shortage of space, food, and resources. Examples of antifertility drugs are Norethindrone and Ethynyl estradiol.
Conceptual Questions(FAQs)
Question 1: Name any two examples of tranquilizers. (This is a universal question in which the drug name will vary)
Answer:
Iproniazid and Phenelzine are two of the tranquillizers.
Question 2: Which drug can be called an ideal drug?
Answer:
The ideal drug is the one that causes least or no side effects to the user and should not disrupt or interrupt the human body’s critical functions, but rather kill the disease-causing pathogen.
Question 3: Which drug acts without loss of consciousness?
Answer:
Analgesics are the drugs used to relieve pain without loss of consciousness.
Question 4: What are the Antimicrobials and their types?
Answer:
Antimicrobials are medications that are produced from plants or microbes and are used to kill or stop the growth of microbes.
Antimicrobials are divided into three categories they are,
- Antibiotic: Used inside the human body
- Antiseptic: Used on the tissue
- Disinfectants: on nonliving things
Question 5: Which drug is used to treat mood swings?
Answer:
Noradrenaline, a neurotransmitter, is used to fix mood swings.
Question 6: What is penicillin?
Answer:
Penicillin is an antibiotic drug which is a drug that is used to treat bacterial infections like pneumonia.
Question 7: Why is Antifertility drug important?
Answer:
The increase in population is a very critical issue that is causing a shortage of resources and hard to fulfill the daily needs and to control the population it is important to have antifertility drugs for family planning.


