Anand Classes explains Positive, Negative, and Zero Overlap of Atomic Orbitals in simple terms with diagrams, solved examples, and detailed explanations. This topic is essential for mastering the concept of orbital overlap in Valence Bond Theory, which directly relates to the strength and formation of chemical bonds. Students preparing for Class 11, Class 12, JEE, and NEET exams will also find well-structured MCQs, Questions-Answers, Assertion-Reason, and Case Study-based problems. Click the print button to download study material and notes.
What Happens When Atomic Orbitals Overlap?
When two atoms approach each other, their atomic orbitals overlap.
- The overlap between different types of s and p orbitals can be positive, negative, or zero depending upon the properties of the overlapping orbitals.
What is Positive Overlap, Negative Overlap and Zero Overlap of Atomic Orbitals ?
Positive Overlap
- When two 2pz orbitals overlap along the internuclear axis with lobes of the same sign, positive overlap occurs.
$$
2p_z \; (\text{lobe +}) \; + \; 2p_z \; (\text{lobe +}) \;\;\longrightarrow\;\; \text{Positive Overlap}
$$
Negetive Overlap
- When two 2pz orbitals overlap along the internuclear axis with lobes of opposite sign, negative overlap occurs.
- Lobes of opposite sign cannot combine.
$$
2p_z \; (\text{lobe +}) \; + \; 2p_z \; (\text{lobe –}) \;\;\longrightarrow\;\; \text{Negative Overlap}
$$
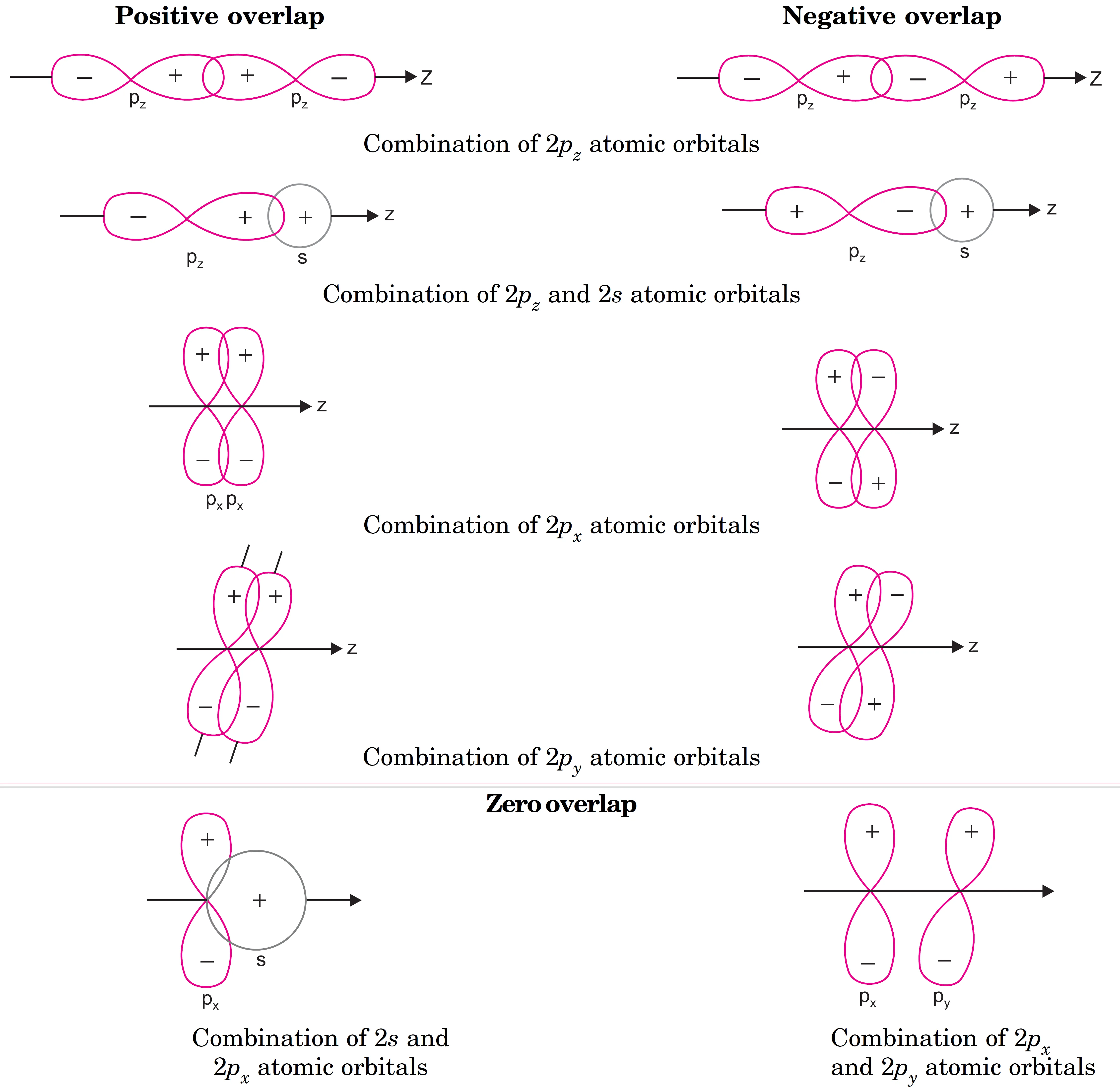
Zero Overlap
- Certain arrangements of s- and p-orbitals do not overlap effectively, leading to zero overlap.
- In this case, no bond formation occurs because the orbitals cannot combine.
Short Answer Conceptual Types Questions (SAT)
Q1. What is orbital overlap?
Answer: Orbital overlap occurs when atomic orbitals of two atoms come close and partially merge, allowing electrons to be shared between atoms.
Q2. Explain positive and negative overlap.
Answer:
- Positive overlap: Lobes of the same phase combine → increases electron density → bond formation.
- Negative overlap: Lobes of opposite phase combine → electron density decreases → antibonding orbital forms.
Q3. What is zero overlap? Give an example.
Answer: Zero overlap occurs when orbitals are oriented unfavorably, so no electron density forms between nuclei.
- Example: An $s$ orbital overlapping sideways with a $p_x$ orbital along the $z$-axis.
Q4. How does the extent of overlap affect bond strength?
Answer: Greater overlap → stronger bond; lesser overlap → weaker bond.
Q5. Why can $\pi$-bonds never exist independently?
Answer: $\pi$-bonds require a $\sigma$-bond to hold atoms together; sidewise overlap alone cannot hold atoms.
Multiple Choice Questions (MCQs)
Q1. Which type of overlap leads to a sigma ($\sigma$) bond?
(a) Sidewise overlap
(b) End-to-end overlap
(c) No overlap
(d) Overlap of $d$ orbitals only
Answer: (b) End-to-end overlap
Explanation: $\sigma$-bonds form by axial (head-on) overlap along the internuclear axis.
Q2. In hydrogen ($H_2$), which type of orbital overlap occurs?
(a) s–s positive overlap
(b) s–p negative overlap
(c) p–p zero overlap
(d) s–p zero overlap
Answer: (a) s–s positive overlap
Explanation: Two $1s$ orbitals overlap end-to-end with the same phase, forming a $\sigma$ bond.
Q3. What happens when two $2p_z$ orbitals of opposite phase overlap?
(a) Positive overlap → strong bond
(b) Negative overlap → antibonding orbital
(c) Zero overlap → no bond
(d) Forms a sigma bond
Answer: (b) Negative overlap → antibonding orbital
Explanation: Lobes of opposite phase cancel out, forming an antibonding orbital ($\sigma^$ or $\pi^$).
Assertion-Reason Type Questions
Q1.
Assertion (A): Positive overlap increases electron density between nuclei.
Reason (R): Constructive interference of orbitals with same phase occurs.
Options:
(a) Both A and R are true, and R is the correct explanation of A
(b) Both A and R are true, but R is not the correct explanation of A
(c) A is true, R is false
(d) A is false, R is true
Answer: (a) Both A and R are true, and R is the correct explanation of A
Q2.
Assertion (A): $\pi$-bonds allow free rotation around the bond axis.
Reason (R): Electron clouds in $\pi$-bonds are symmetrically distributed along the internuclear axis.
Answer: (d) A is false, R is true
Explanation: Free rotation is not possible around a $\pi$-bond because electron clouds lie above and below the plane, restricting rotation.
Case Study
Passage:
Two atoms approach each other, and their atomic orbitals begin to overlap. Depending on the orientation and phases of the orbitals, positive, negative, or zero overlap can occur. For example, two $2p_z$ orbitals overlapping along the internuclear axis with lobes of the same phase show positive overlap. A $\pi$-bond always forms in addition to a $\sigma$-bond and involves sidewise overlap.
Q1. What type of overlap occurs when two $2p_z$ orbitals with the same phase interact?
Answer: Positive overlap → bonding orbital forms.
Q2. What happens if the lobes of the $2p_z$ orbitals have opposite phase?
Answer: Negative overlap → antibonding orbital ($\sigma^$ or $\pi^$) forms.
Q3. Can a $\pi$-bond exist without a $\sigma$-bond? Explain.
Answer: No. A $\pi$-bond requires a $\sigma$-bond to hold atoms along the internuclear axis because sidewise overlap alone is insufficient.
Q4. Which type of bond is stronger: $\sigma$ or $\pi$? Why?
Answer: $\sigma$-bond, because end-to-end overlap allows greater electron density between nuclei than sidewise overlap in a $\pi$-bond.


