Anand Classes provides in-depth Class 11 Chemistry study material for JEE and NEET on Lewis Symbols or Electron Dot Symbols, explaining their significance with step-by-step solved examples, MCQs, assertion-reason questions, and case study-based problems to help students understand the representation of valence electrons and chemical bonding concepts effectively. Click the print button to download study material and notes.
What are Lewis Symbols ?
In the formation of a molecule, only the outer shell electrons take part in chemical combination. These are called valence shell electrons. Inner shell electrons generally do not take part in bonding.
G.N. Lewis introduced notations to represent valence electrons called Lewis symbols or electron dot symbols.
Lewis Symbols or Electron Dot Symbols
- The symbol of the element represents the atom except the valence electrons.
- The valence electrons are represented by placing dots ($\cdot$) or crosses ($\times$) around the symbol.
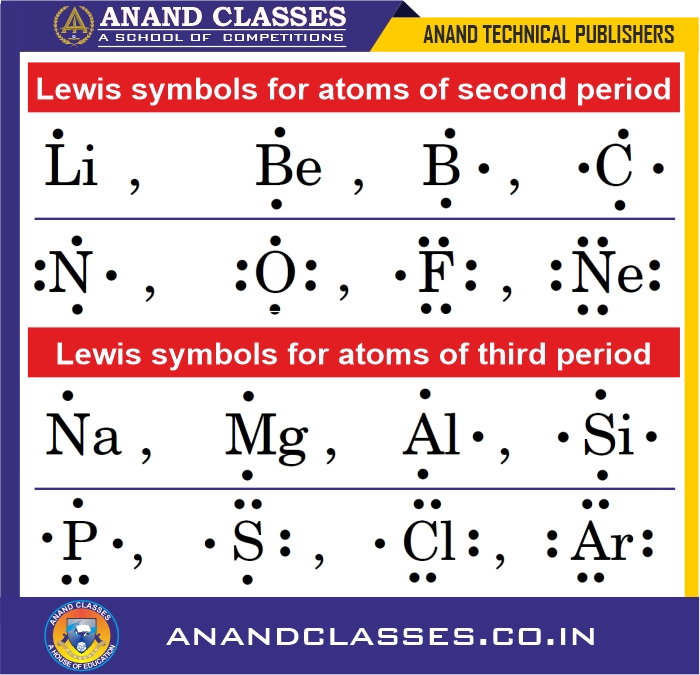
Example: Lewis symbols for second-period and third-period elements:
Significance of Lewis Symbols
- Indicate the number of valence electrons: Lewis symbols show the number of electrons in the outermost shell.
- These help to Predict common or group valence of the element:
- Lithium ($Li$) → 1 valence electron → monovalent
- Beryllium ($Be$) → 2 valence electrons → divalent
- Boron ($B$) → 3 valence electrons → trivalent
- Carbon ($C$) → 4 valence electrons → tetravalent
For N, O, F, Ne, the common valence is calculated as:
Common valence = 8 − number of valence electrons
- Nitrogen ($N$): 3
- Oxygen ($O$): 2
- Fluorine ($F$): 1
- Neon ($Ne$): 0
The common valence of an element is either equal to the number of dots in the Lewis symbol or 8 minus the number of dots.
Solved Examples on Lewis Symbols
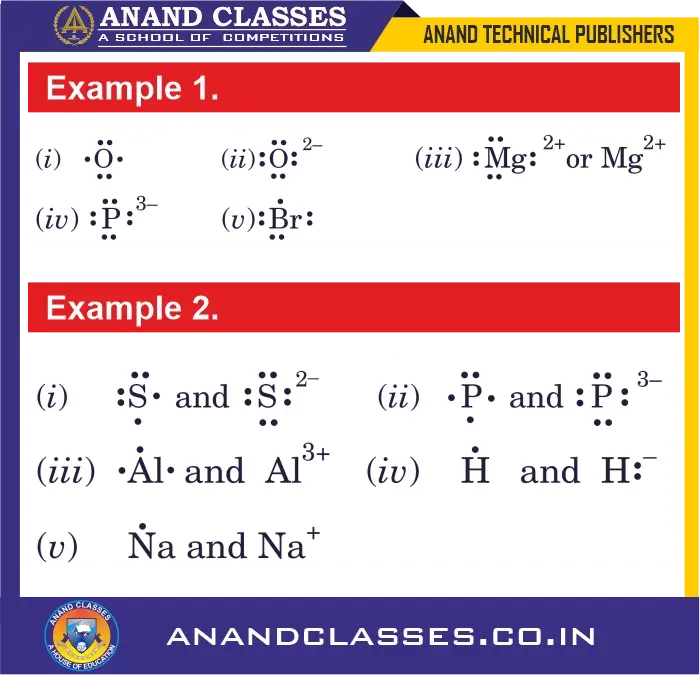
Example 1.
Write Lewis dot symbols for the following atoms and ions :
(i) O (ii) O$^{2-}$ (iii) Mg$^{2+}$ (iv) P$^{3-}$ (v) Br
Solution:
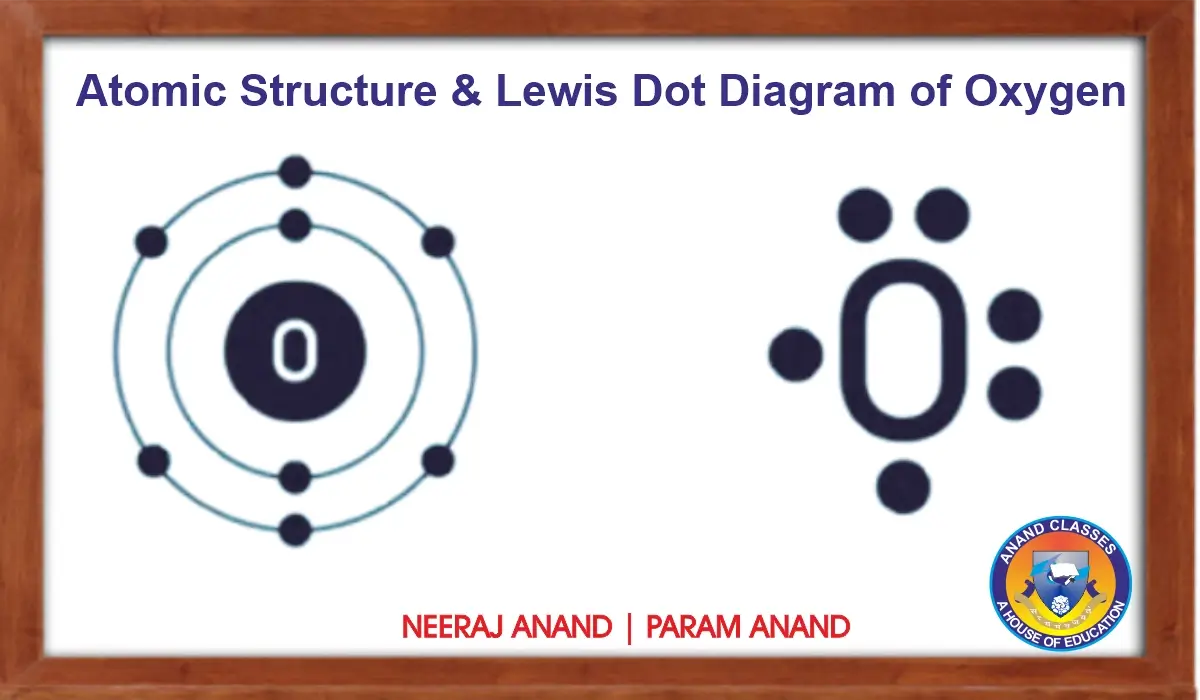
(i) Oxygen (O): Atomic number = 8.
Electronic configuration = $1s^2 2s^2 2p^4$.
Valence electrons = 6 → Lewis symbol = $O$ with 6 dots.
(ii) Oxide ion (O$^{2-}$): Oxygen gains 2 electrons.
Valence electrons = $6 + 2 = 8$ → Lewis symbol = $O^{2-}$ with 8 dots.
(iii) Magnesium ion (Mg$^{2+}$): Magnesium (Z = 12) has electronic configuration $1s^2 2s^2 2p^6 3s^2$.
Valence electrons = 2. After losing 2, Mg$^{2+}$ has 0 valence electrons in its outermost shell → Lewis symbol = $Mg^{2+}$ with no dots.
(iv) Phosphide ion (P$^{3-}$): Phosphorus (Z = 15), configuration $1s^2 2s^2 2p^6 3s^2 3p^3$.
Valence electrons = 5. After gaining 3 electrons → 8 valence electrons.
Lewis symbol = $P^{3-}$ with 8 dots.
(v) Bromine (Br): Atomic number = 35.
Configuration = $[Ar] 3d^{10} 4s^2 4p^5$.
Valence electrons = 7 → Lewis symbol = $Br$ with 7 dots.
Final Answer: The Electron dot structures are shown in following diagram in next question diaram of solution
Example 2.
Write Lewis dot symbols for the following atoms and ions:
(i) S and S²⁻ (ii) P and P³⁻ (iii) Al and Al³⁺ (iv) H and H⁻ (v) Na and Na⁺
Answer:
(i) Sulphur (S) and Sulphide ion (S²⁻):
- S: Atomic number 16 → 6 valence electrons. Lewis symbol: $\mathrm{S}$ with 6 dots.
- S²⁻: Gains 2 electrons → 8 valence electrons. Lewis symbol: $\mathrm{S^{2-}}$ with 8 dots.
(ii) Phosphorus (P) and Phosphide ion (P³⁻):
- P: Atomic number 15 → 5 valence electrons. Lewis symbol: $\mathrm{P}$ with 5 dots.
- P³⁻: Gains 3 electrons → 8 valence electrons. Lewis symbol: $\mathrm{P^{3-}}$ with 8 dots.
(iii) Aluminium (Al) and Aluminium ion (Al³⁺):
- Al: Atomic number 13 → 3 valence electrons. Lewis symbol: $\mathrm{Al}$ with 3 dots.
- Al³⁺: Loses 3 electrons → 0 valence electrons. Lewis symbol: $\mathrm{Al^{3+}}$ with no dots.
(iv) Hydrogen (H) and Hydride ion (H⁻):
- H: Atomic number 1 → 1 valence electron. Lewis symbol: $\mathrm{H}$ with 1 dot.
- H⁻: Gains 1 electron → 2 electrons (duplet). Lewis symbol: $\mathrm{H^-}$ with 2 dots.
(v) Sodium (Na) and Sodium ion (Na⁺):
- Na: Atomic number 11 → 1 valence electron. Lewis symbol: $\mathrm{Na}$ with 1 dot.
- Na⁺: Loses 1 electron → 0 valence electrons. Lewis symbol: $\mathrm{Na^+}$ with no dots.
Multiple Choice Questions (MCQs) on Lewis Symbols
Q1. Which statement about Lewis symbols is correct?
(a) They represent all the electrons of an atom
(b) They represent only the valence electrons as dots around the symbol
(c) They show the arrangement of electrons in shells
(d) They are used to indicate nuclear charge
Answer: (b)
Explanation: Lewis symbols represent only the valence electrons by placing dots or crosses around the element’s symbol.
Q2. How many dots are placed around the symbol of nitrogen in its Lewis symbol?
(a) 3
(b) 5
(c) 7
(d) 8
Answer: (b)
Explanation: Nitrogen has the electronic configuration $1s^2 2s^2 2p^3$, i.e., 5 valence electrons, so its Lewis symbol has 5 dots.
Q3. Which of the following elements has a Lewis symbol with 8 dots?
(a) Fluorine
(b) Neon
(c) Oxygen
(d) Nitrogen
Answer: (b)
Explanation: Neon ($1s^2 2s^2 2p^6$) has a completely filled valence shell with 8 electrons, so its Lewis symbol has 8 dots.
Q4. The common valence of oxygen, as predicted by its Lewis symbol, is:
(a) 6
(b) 2
(c) 4
(d) 8
Answer: (b)
Explanation: Oxygen has 6 valence electrons. Its valence = $8 – 6 = 2$.
Q5. Which element has the same number of dots in its Lewis symbol as its group number in the periodic table?
(a) Neon
(b) Nitrogen
(c) Oxygen
(d) Boron
Answer: (d)
Explanation: Boron (group 13) has 3 valence electrons and its Lewis symbol has 3 dots, equal to its group valency.
Assertion–Reason Questions on Lewis Symbols
Q6. Assertion (A): Lewis symbols represent only valence electrons.
Reason (R): Inner shell electrons do not participate in chemical bonding.
Options:
(a) Both A and R are true, and R is the correct explanation of A
(b) Both A and R are true, but R is not the correct explanation of A
(c) A is true, but R is false
(d) A is false, but R is true
Answer: (a)
Explanation: Valence electrons are represented because they alone participate in bonding, while inner electrons remain unaffected.
Q7. Assertion (A): Neon has zero valency.
Reason (R): Neon has 8 valence electrons, which makes its shell stable.
Options:
(a) Both A and R are true, and R is the correct explanation of A
(b) Both A and R are true, but R is not the correct explanation of A
(c) A is true, but R is false
(d) A is false, but R is true
Answer: (a)
Explanation: Neon already has a stable octet, so it does not combine with other atoms → valency = 0.
Q8. Assertion (A): Oxygen is divalent.
Reason (R): Oxygen has 6 valence electrons, and its common valence is $8 – 6 = 2$.
Options:
(a) Both A and R are true, and R is the correct explanation of A
(b) Both A and R are true, but R is not the correct explanation of A
(c) A is true, but R is false
(d) A is false, but R is true
Answer: (a)
Explanation: The valence of oxygen is calculated from its Lewis symbol → divalent.
Case Study on Lewis Symbols
Case Study Passage
Lewis symbols, introduced by G.N. Lewis, are used to represent valence electrons as dots around an element’s symbol. They are useful in predicting the valency of an element. For Li, Be, B, and C, the valency is equal to the number of dots (valence electrons). For N, O, F, and Ne, the valency is equal to $8 -$ (number of dots). For example, oxygen with 6 dots is divalent, and fluorine with 7 dots is monovalent.
Q9. Which of the following correctly represents the Lewis symbol of fluorine?
(a) F with 5 dots
(b) F with 7 dots
(c) F with 8 dots
(d) F with 6 dots
Answer: (b)
Explanation: Fluorine has 7 valence electrons ($2s^2 2p^5$), so its Lewis symbol has 7 dots.
Q10. What is the valency of nitrogen as predicted by its Lewis symbol?
(a) 5
(b) 2
(c) 3
(d) 8
Answer: (c)
Explanation: Nitrogen has 5 valence electrons. Its valency = $8 – 5 = 3$.
Q11. Which of the following is true regarding Lewis symbols?
(a) They help predict the number of protons in the nucleus
(b) They help predict the valency of elements
(c) They represent the entire electron configuration
(d) They are used only for noble gases
Answer: (b)
Explanation: Lewis symbols are mainly used to predict valency by showing the number of valence electrons.