Anand Classes explains the Differences Between Bonding and Antibonding Molecular Orbitals in a simple and conceptual way, including the formation and characteristics of σ (sigma) and π (pi) molecular orbitals. Students will learn how atomic orbitals combine to form molecular orbitals through constructive and destructive interference, and how this affects the stability, energy, and bond formation in molecules. This topic is essential for understanding the Molecular Orbital Theory (MOT) and is supported with MCQs, Q&A, Assertion Reason, and Case Study questions for Class 11, Class 12, JEE, and NEET chemistry exams. Click the print button to download study material and notes.
What are the Relative Energies of Bonding and Antibonding Molecular Orbitals?
We have learnt that in the case of bonding molecular orbital, the attraction of both the nuclei for both the electrons is increased. This results in lowering of energy.
In the case of antibonding molecular orbital, the electrons try to go away from the nuclei and this corresponds to a repulsive state. The energy of this orbital will be higher.
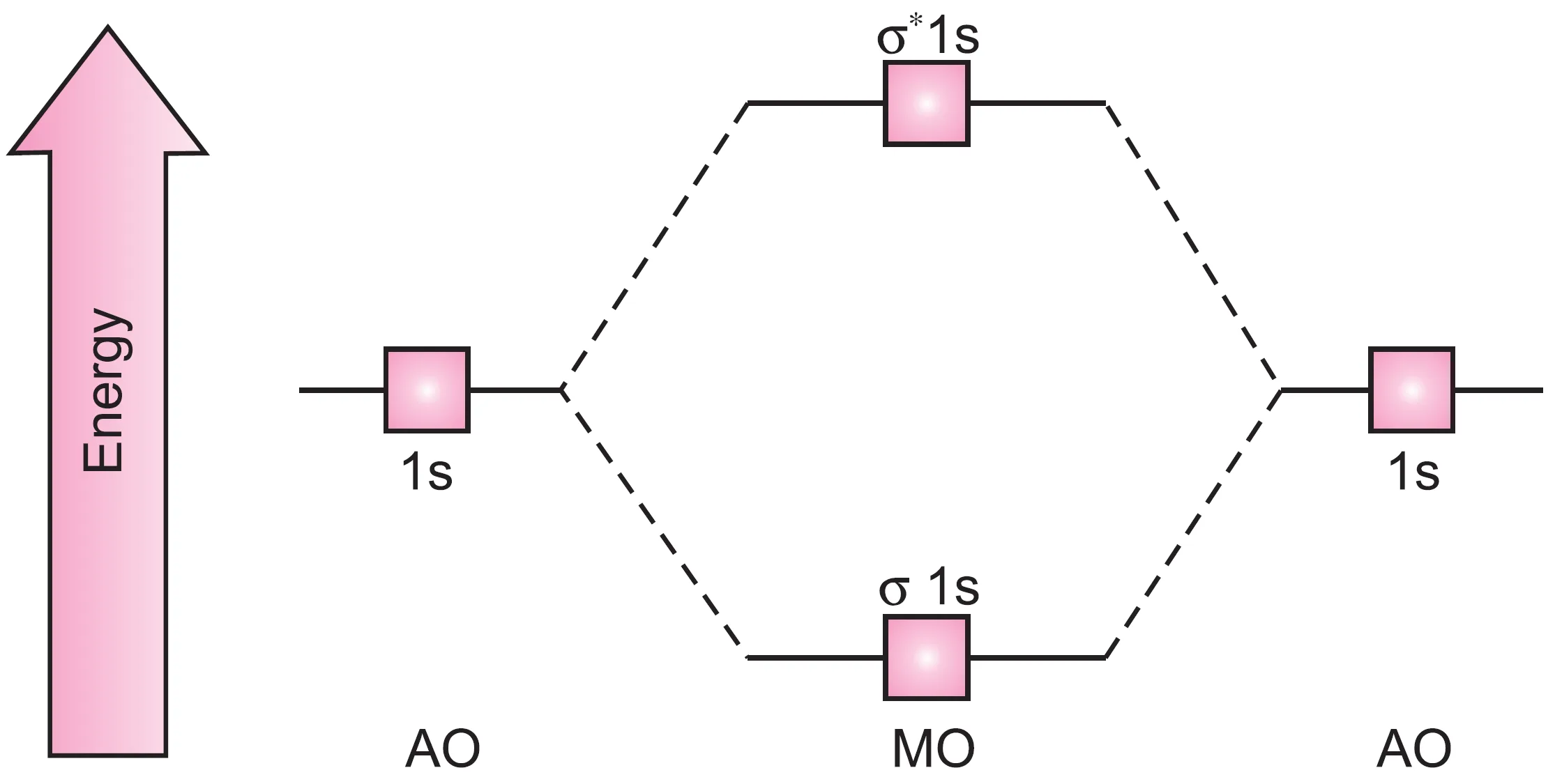
The relative energies of bonding and antibonding molecular orbitals along with atomic orbitals can be represented as shown in Figure..
orbitals
Thus, the bonding molecular orbital is stabilized relative to the energy of the isolated atoms and antibonding molecular orbital is destabilized relative to the individual atoms.
Further, it may be noted that the bonding MO is stabilized to the same extent as the antibonding MO is destabilized.
What are the Differences Between Bonding and Antibonding Molecular Orbitals?
| Feature | Bonding Molecular Orbital (MO) | Antibonding Molecular Orbital (MO) |
|---|---|---|
| Formation | Formed by addition of overlapping of atomic orbitals | Formed by subtraction of overlapping of atomic orbitals |
| Wave Function | $$\psi(MO) = \psi_A + \psi_B$$ | $$\psi^*(MO) = \psi_A – \psi_B$$ |
| Sign of Lobes | Formed when lobes of combining atomic orbitals have same sign | Formed when lobes of combining atomic orbitals have opposite sign |
| Electron Density | Greater electron density in the region between the two nuclei | Lesser electron density in the region between the two nuclei |
| Forces on Nuclei | Forces tend to bring the nuclei together → attraction | Forces tend to push the nuclei apart → repulsion |
| Contribution to Bonding | Electrons in bonding MO stabilize the molecule | Electrons in antibonding MO destabilize the molecule |
| Energy Level | Lower energy than isolated atomic orbitals | Higher energy than isolated atomic orbitals |
How Do 2s and 2p Atomic Orbitals Combine to Form Molecular Orbitals?
Combination of 2s atomic orbitals :
Like 1s-orbitals, 2s-orbitals combine by addition and subtraction of overlapping to form bonding and antibonding molecular orbitals. These are labelled as σ2s and σ*2s.
These molecular orbitals have exactly the same shapes as σ1s and σ*1s MOs but they are slightly larger in size.
Combination of 2p atomic orbitals :
There are three p-orbitals namely 2px, 2py and 2pz which are directed in space along the x, y and z axes respectively.
By convention, we assume z-axis as the internuclear axis. The x and y axes are perpendicular to it.
The combination of 2p-orbitals forms two types of MOs:
- Sigma (σ) MOs
- Pi (π) MOs
How are σ-Molecular Orbitals Formed?
When two 2pz orbitals are brought closer along the internuclear axis, they overlap head-on forming a σ-bond.
- When the two orbitals overlap by the addition of electron waves (++ lobes overlap), a bonding molecular orbital is formed (σ2pz).
- When the two wave functions are subtracted, an antibonding molecular orbital is formed (σ*2pz).
Thus, two 2pz atomic orbitals combine to form two molecular orbitals (σ2pz and σ*2pz) :
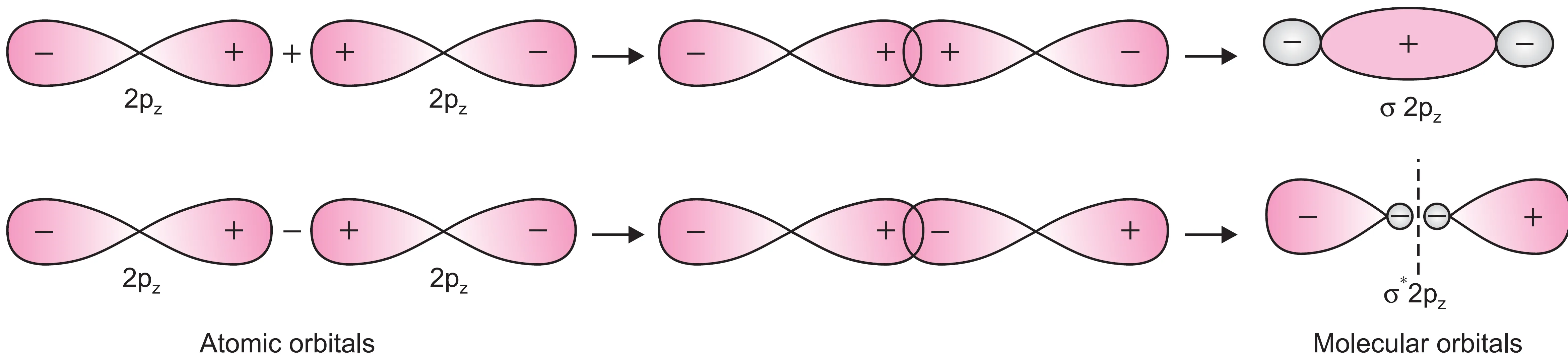
- A bonding MO (σ2pz)
- An antibonding MO (σ*2pz)
How are π-Molecular Orbitals Formed?
When a 2px orbital of one atom approaches the 2px orbital of another atom, the overlapping occurs sidewise (lateral overlap) and not end-to-end.
- The molecular orbital formed by this sidewise overlap is called a pi (π) molecular orbital.
- The molecular orbital formed is not symmetrical about the internuclear axis.
- Overlapping by addition of electron waves → bonding π molecular orbital (π2px).
- Overlapping by subtraction of electron waves → antibonding π molecular orbital (π*2px).
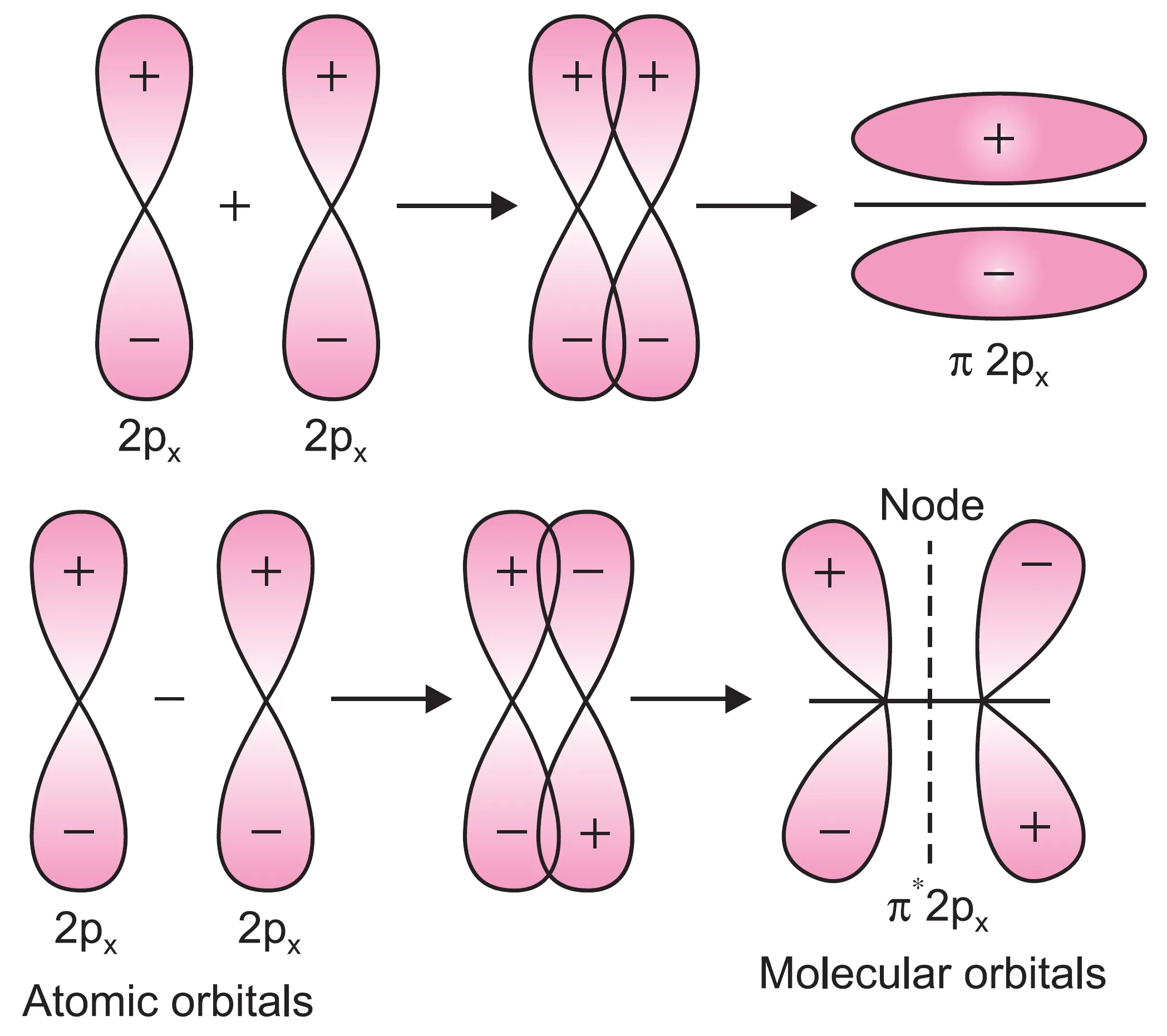
molecular orbitals from 2px atomic orbitals.
Similarly, when 2py orbitals overlap, they form π2py and π*2py which are exactly similar to π2px and π*2px.
What are the Differences Between σ and π Molecular Orbitals?
| Feature | σ Molecular Orbital (σ MO) | π Molecular Orbital (π MO) |
|---|---|---|
| Type of Overlap | Formed by head-to-head overlap of atomic orbitals along the internuclear axis | Formed by sidewise overlap of atomic orbitals perpendicular to the internuclear axis |
| Overlap Region | Maximum overlap region | Minimum overlap region |
| Symmetry | Orbital is symmetrical to rotation about internuclear axis | Orbital is not symmetrical to rotation about internuclear axis |
| Bond Strength | Leads to formation of a strong bond | Leads to formation of a weak bond |
What are the Conditions for the Combination of Atomic Orbitals?
Molecular orbitals are formed by the combination of atomic orbitals, but not all orbitals can combine.
The main conditions are:
- The combining atomic orbitals must have same or nearly the same energies → For example, in case of homonuclear diatomic molecules of the type A2, 2s-orbital of one atom can combine with 2s-orbital of another atom but 1s-orbital of one atom cannot combine with 2s-orbital of another atom.
- The extent of overlapping between the atomic orbitals of two atoms should be large → Greater the extent of overlap, the greater will be the electron density between the nuclei of a molecular orbital.
- The combining atomic orbitals must have the same symmetry (or proper orientation) about the moecular axis → For example, if we take z-axis as the internuclear axis, 2pz orbital of one atom can combine with 2pz orbital of other atom but 2pz orbital of one atom cannot combine with 2px or 2py orbitals of other atom because of their different orientations or symmetries.
- 2pz can combine with 2pz (internuclear axis = z-axis).
- 2pz cannot combine with 2px or 2py due to different orientations.
- 2s can combine with 2pz but not with 2px or 2py.
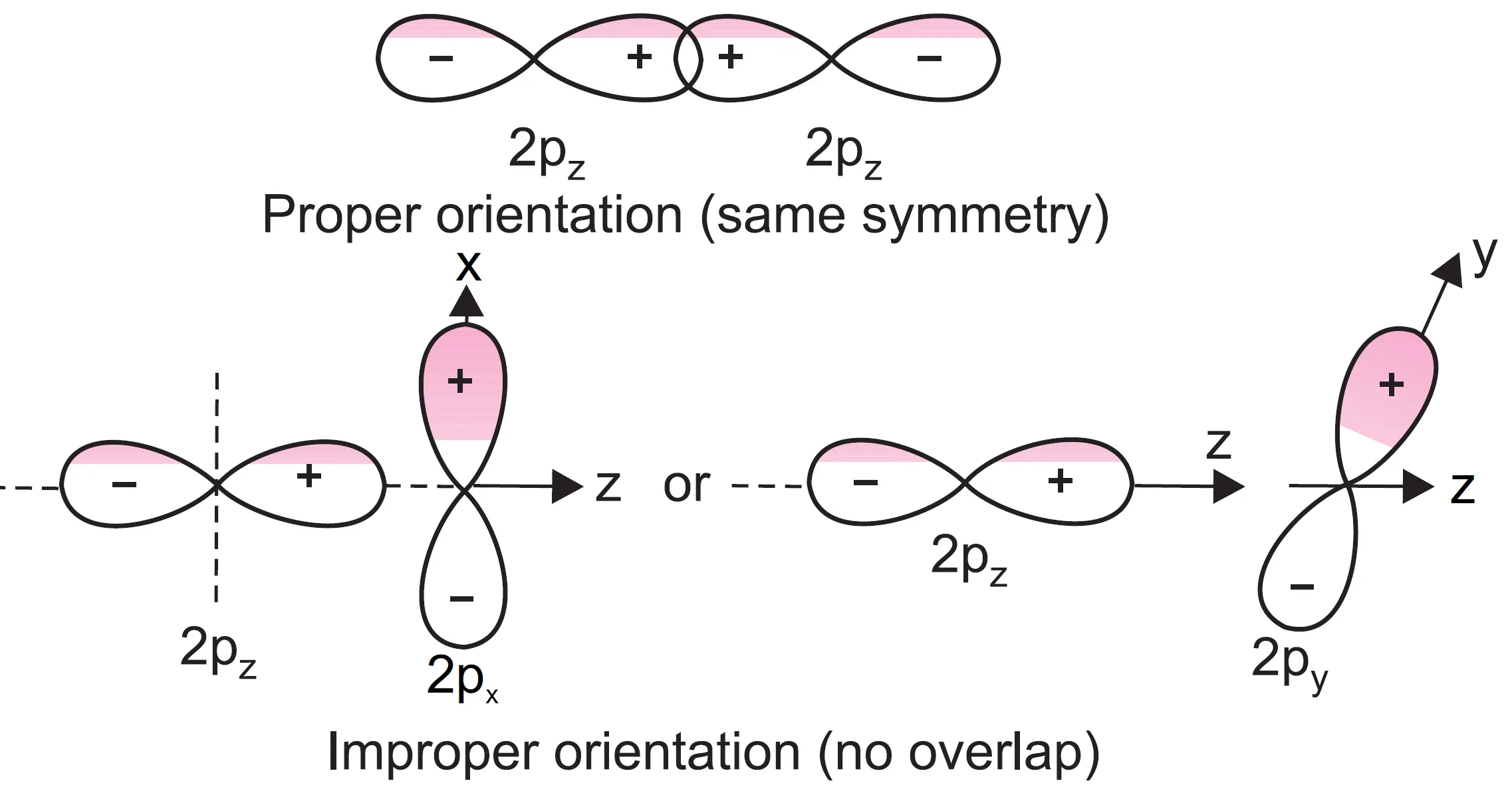
impossibility of combination of 2pz and 2px or 2pz and 2py.
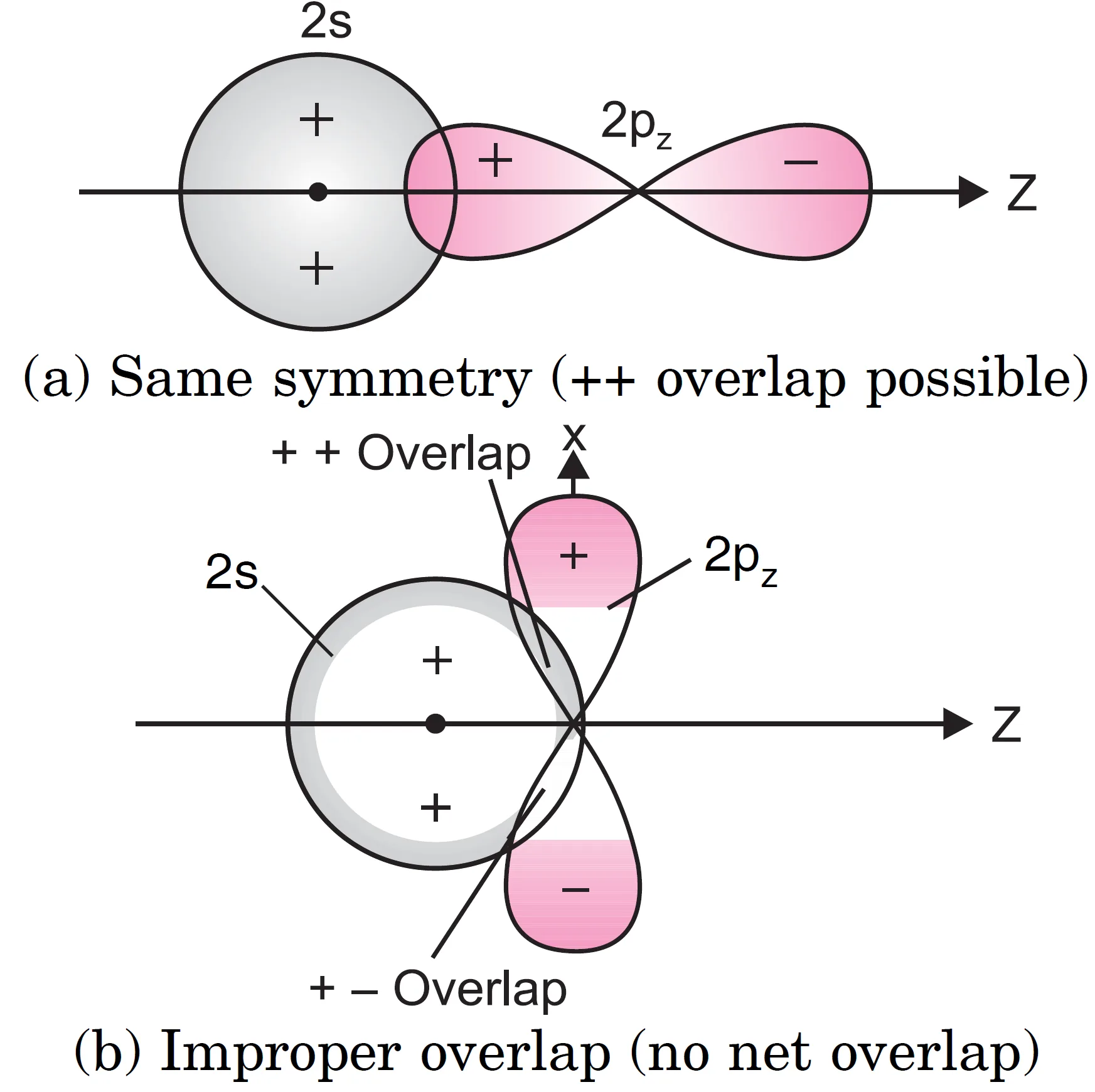
Similarly a 2s-orbital of one atom can combine with 2pz orbital of another atom [see Figure] but it cannot overlap with 2px or 2py orbital of another atom. When 2s-orbital of one atom A overlaps with 2px orbital of another atom B [see Figure)], ++ overlap is cancelled by the + – overlap. As a result, no molecular orbital is formed. In other words, such a combination (2s with
2px or 2s with 2py) is not possible.
Question : Label the molecular orbitals formed by the following combinations of atomic orbitals (Assume z-axis as internuclear axis):
(i) 2s + 2s
(ii) 2px – 2px
(iii) 2pz + 2pz
(iv) 1s – 1s
(v) 2py + 2py
Solution
- (i) 2s + 2s → σ2s
- (ii) 2px – 2px → π*2px
- (iii) 2pz + 2pz → σ2pz
- (iv) 1s – 1s → σ*1s
- (v) 2py + 2py → π2py
Practice Questions on Molecular Orbital Theory
Short Answer Conceptual Types (SAT)
Q1. Why is the energy of a bonding molecular orbital lower than that of atomic orbitals?
Answer: Because in a bonding MO, the attraction of both nuclei for electrons increases, leading to greater electron density between nuclei. This stabilizes the molecule and lowers its energy.
Q2. Why do antibonding orbitals have higher energy?
Answer: In antibonding MO, electron density is low between the nuclei and electrons are pushed away, producing repulsion. This destabilizes the molecule, raising energy above that of atomic orbitals.
Q3. Write the wave functions of bonding and antibonding molecular orbitals.
Answer:
- Bonding MO: $$\psi(MO) = \psi_A + \psi_B$$
- Antibonding MO: $$\psi^*(MO) = \psi_A – \psi_B$$
Q4. Why are σ-bonds stronger than π-bonds?
Answer: Because σ-bonds are formed by head-on overlap which has maximum electron density along internuclear axis, whereas π-bonds are formed by sidewise overlap, which is weaker.
Multiple Choice Questions (MCQs)
Q1. Which of the following is true for a bonding molecular orbital?
(a) Electron density is concentrated away from nuclei
(b) Electrons cause repulsion between nuclei
(c) Energy is lower than atomic orbitals
(d) Formed by destructive interference of wave functions
Answer: (c) Energy is lower than atomic orbitals
Explanation: Bonding MOs stabilize molecules by lowering energy due to increased electron density between nuclei.
Q2. The wave function of an antibonding MO is:
(a) $\psi_A + \psi_B$
(b) $\psi_A – \psi_B$
(c) $\psi_A \times \psi_B$
(d) None of these
Answer: (b) $\psi_A – \psi_B$
Explanation: Antibonding orbitals are formed by subtraction (destructive overlap) of atomic orbitals.
Q3. Which type of overlap gives maximum bond strength?
(a) Sidewise overlap of p-orbitals
(b) Head-on overlap of orbitals
(c) Overlap of orbitals with different symmetry
(d) No overlap
Answer: (b) Head-on overlap of orbitals
Explanation: Head-on overlap forms σ bonds, which are stronger than π bonds formed by sidewise overlap.
Q4. In π molecular orbitals, electron density is concentrated:
(a) On the internuclear axis
(b) Above and below the internuclear axis
(c) Inside the nucleus
(d) Randomly distributed
Answer: (b) Above and below the internuclear axis
Explanation: π MOs are formed by lateral overlap, hence electron cloud lies above and below the bond axis.
Assertion-Reason Type Questions
Q1.
Assertion (A): Bonding molecular orbitals stabilize the molecule.
Reason (R): Electron density is concentrated between the two nuclei.
- (a) Both A and R are true, and R is the correct explanation of A.
- (b) Both A and R are true, but R is not the correct explanation of A.
- (c) A is true, R is false.
- (d) A is false, R is true.
Answer: (a) Both A and R are true, and R is the correct explanation of A.
Explanation: Stabilization in bonding MO is due to high electron density between nuclei which holds them together.
Q2.
Assertion (A): Antibonding orbitals are more stable than bonding orbitals.
Reason (R): Antibonding orbitals have greater electron density between nuclei.
- (a) Both A and R are true, and R is the correct explanation of A.
- (b) Both A and R are true, but R is not the correct explanation of A.
- (c) A is true, R is false.
- (d) A is false, R is true.
Answer: (d) A is false, R is true.
Explanation: Antibonding orbitals are less stable (not more) because electron density between nuclei is low, not high.
Case Study
Passage:
Consider two hydrogen atoms combining to form H₂. The atomic orbitals (1s and 1s) combine to form two molecular orbitals: σ1s (bonding) and σ*1s (antibonding). Electrons prefer to occupy the lower energy bonding orbital first. If both electrons occupy the bonding orbital, the molecule is stable. If electrons occupy both bonding and antibonding orbitals, the bond cancels out and the molecule is unstable.
Questions:
- Which molecular orbitals are formed when 1s orbitals of two H atoms combine?
- Why is H₂ molecule stable?
- Why is He₂ molecule unstable?
- What is the bond order of H₂ and He₂?
Answers:
- σ1s (bonding) and σ*1s (antibonding) orbitals.
- H₂ is stable because both electrons occupy the σ1s bonding orbital.
- He₂ is unstable because it has 2 electrons in σ1s and 2 in σ*1s, so bonding and antibonding cancel.
- Bond order = ½ (Nb – Na).
- For H₂: = ½ (2 – 0) = 1 → stable
- For He₂: = ½ (2 – 2) = 0 → unstable