Anand Classes provides detailed Class 11 Chemistry notes on Valence Shell Electron Pair Repulsion (VSEPR) Theory with complete postulates explaining the role of lone pairs and bond pairs in determining molecular geometry. Learn how the repulsion between electron pairs influences bond angles and shapes of molecules such as BeCl₂, BF₃, CH₄, NH₃, and H₂O. These well-structured notes include diagrams, solved examples, Q&A, MCQs, and important points for NEET, JEE, and CBSE board exams. Click the print button to download study material and notes.
What is the Geometry or Shape of Molecules?
The different atoms in a molecule have definite geometric arrangements in space around the central atom. This definite relative arrangement of the bonded atoms in a molecule is known as the geometry or shape of the molecules.
What is the Valence Shell Electron Pair Repulsion (VSEPR) Theory?
In 1940, Sidgwick and Powell proposed a simple theory based on the repulsive interactions of the electron pairs in the valence shell of the atoms. It was further developed and refined by Nyholm and Gillespie in 1957.
They suggested that the shapes of molecules can be determined by the number of electron pairs (bonding as well as non-bonding) in the valence shell of the central atom and proposed a theory known as Valence Shell Electron Pair Repulsion (VSEPR) theory.
The basic idea of this theory is that bonded atoms in a molecule adopt that particular arrangement in space around the central atom which keeps them on an average as far apart as possible.
What are the Postulates of VSEPR Theory?
- The shape of a molecule depends upon the number of valence shell electron pairs (whether bonded or not) around the central atom.
- In the formation of a bond, the central atom shares its valence electrons with the surrounding atoms.
- In certain cases, not all valence shell electrons take part in bond formation.
- The electrons left in the valence shell without forming bonds exist as lone pairs.
- In methane ($CH_{4}$), carbon uses all four valence electrons in forming four bond pairs.
- In water ($H_{2}O$), oxygen has six valence electrons ($1s^{2} 2s^{2} 2p^{4}$). Hydrogen atoms share two of these six electrons, leaving two lone pairs.
- Thus, in methane → four bond pairs around carbon.
- In water → two bond pairs + two lone pairs around oxygen.
$O$: (Two bond pairs + Two lone pairs)
- Electron pairs in the valence shell repel one another because their electron clouds are negatively charged.
- The electron pairs occupy positions in space that minimize repulsions, staying as far apart as possible to acquire minimum energy (maximum stability).
- The valence shell is taken as a sphere, with electron pairs localized on the spherical surface at maximum distances from one another.
- Electron pairs in multiple bonds are treated as a single unit.
- A double bond (2 pairs) or triple bond (3 pairs) is considered as one super pair equivalent to a single bond pair.
- The repulsion order between different pairs of electrons is: $$ \text{Lone pair–Lone pair} > \text{Lone pair–Bond pair} > \text{Bond pair–Bond pair} $$ The presence of lone pairs causes distortions in the regular geometry of molecules.
- Repulsive forces decrease sharply with increasing angle between electron pairs:
- Strongest at $90^{\circ}$
- Weaker at $120^{\circ}$
- Weakest at $180^{\circ}$
How Did Nyholm and Gillespie Improve the VSEPR Model?
Nyholm and Gillespie (1957) pointed to the important difference between lone pairs and bond pairs.
- The orbital of a bonded pair is under the influence of two nuclei, so most of the electron cloud lies between the nuclei.
- A lone pair is under the influence of only one nucleus, so its electron cloud is spread out and occupies more space.
Example: In ammonia ($NH_{3}$), there are three bond pairs and one lone pair.
- The lone pair is flatter, occupies more space, and repels bond pairs more strongly.
- Since the lone pair is flatter and occupies more space around the central atom, it can interact more and, therefore, it will repel the electron pairs in the neighbouring orbitals strongly than do the electrons in the bonded orbital. Obviously, the repulsion between two lone pairs would be the largest and will be lesser if one of them is bond pair and the least if both are bond pairs.
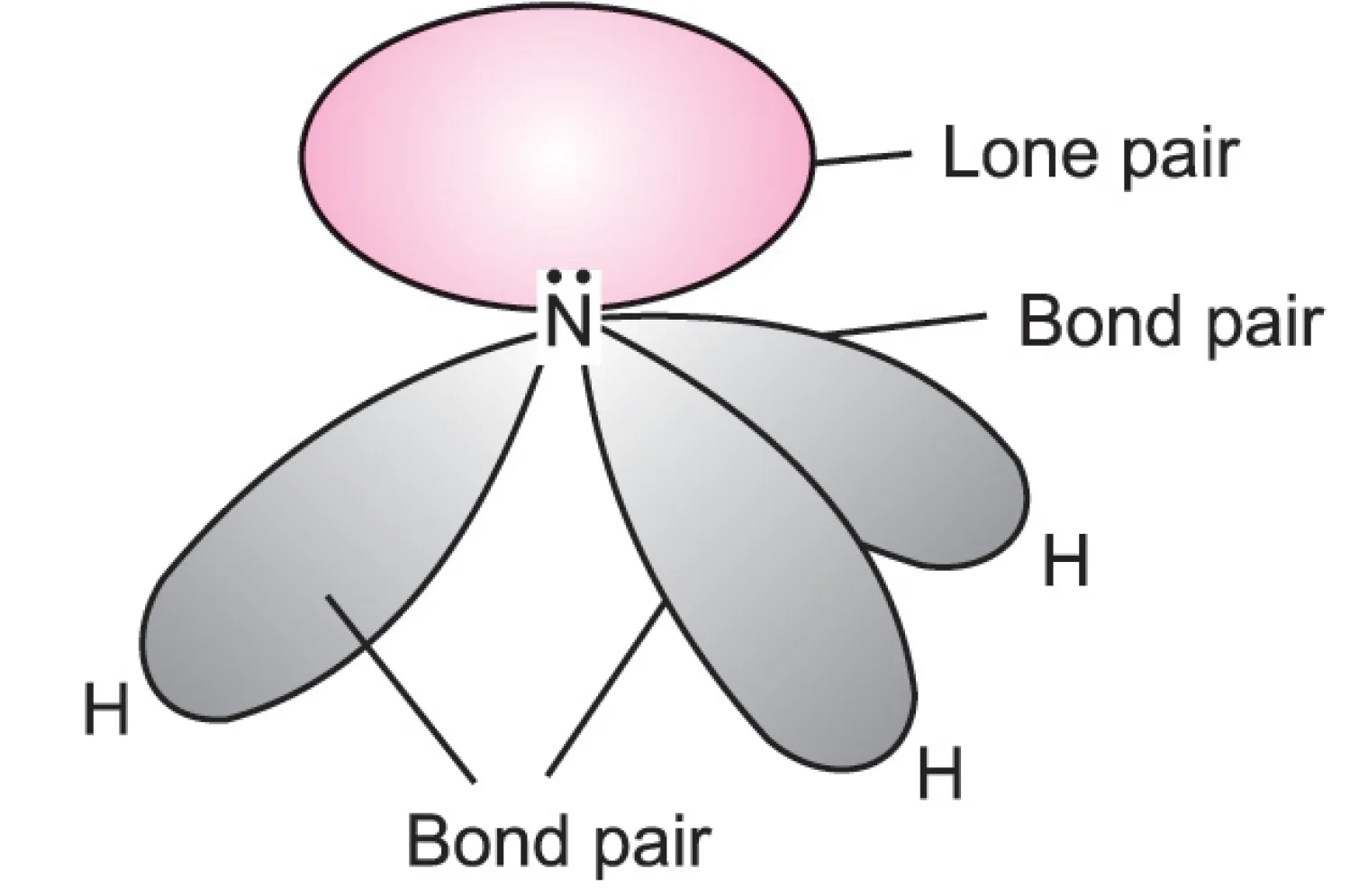
- Hence, lone pair–lone pair repulsion > lone pair–bond pair repulsion > bond pair–bond pair repulsion.
Short Answer Conceptual Type Questions (SAT) on VSEPR Theory
Q1. Why does NH3 have a bond angle of 107o instead of 109.5o?
Answer: In NH3, the central nitrogen atom has 3 bond pairs and 1 lone pair. The lone pair exerts greater repulsion on bond pairs, compressing the bond angle to 107o (from the tetrahedral 109.5o.
Q2. Why is H2O a bent molecule and not linear?
Answer: Oxygen in H2O has 2 bond pairs and 2 lone pairs. The repulsion between lone pairs pushes the bond pairs closer, giving a bent/angular shape with a bond angle of 104.5o.
Q3. Why does BeCl2 have a linear shape?
Answer: In BeCl2, beryllium has 2 bond pairs and no lone pairs, so the electron pairs are 180o apart, giving a linear geometry.
Multiple Choice Questions (MCQs) on VSEPR Theory
Q1. Which of the following has a linear shape?
(a) CO2
(b) SO2
(c) H2O
(d) NH3
Answer: (a) CO2
Explanation: CO2 has two double bonds with no lone pairs on central atom → linear (180o).
Q2. Which one has the smallest bond angle?
(a) CH4 (109.5o)
(b) NH3 (107o)
(c) H2O (104.5o)
(d) CO2 (180o)
Answer: (c) H2O
Explanation: More lone pairs → greater repulsion → bond angle decreases. H2O has 2 lone pairs, giving smallest bond angle (104.5o).
Q3. According to VSEPR theory, the correct order of repulsion is:
(a) Bond pair–Bond pair > Lone pair–Bond pair > Lone pair–Lone pair
(b) Lone pair–Bond pair > Bond pair–Bond pair > Lone pair–Lone pair
(c) Lone pair–Lone pair > Lone pair–Bond pair > Bond pair–Bond pair
(d) Bond pair–Bond pair > Lone pair–Lone pair > Lone pair–Bond pair
Answer: (c)
Explanation: Repulsions decrease in the order:
Lone pair–Lone pair > Lone pair–Bond pair > Bond pair–Bond pair
Assertion–Reason Type Questions on VSEPR Theory
Q1.
Assertion (A): NH3 is pyramidal in shape.
Reason (R): Nitrogen has one lone pair and three bond pairs.
- (a) Both A and R are true, and R is the correct explanation of A.
- (b) Both A and R are true, but R is not the correct explanation of A.
- (c) A is true but R is false.
- (d) A is false but R is true.
Answer: (a)
Explanation: Lone pair on nitrogen pushes the three bond pairs down, giving pyramidal shape.
Q2.
Assertion (A): CO2 is a linear molecule.
Reason (R): The central carbon atom has no lone pairs.
Answer: (a) Both A and R are true, and R is the correct explanation of A.
Q3.
Assertion (A): H2O is more angular than NH3.
Reason (R): Oxygen in H2O has 2 lone pairs, while nitrogen in NH3 has only 1 lone pair.
Answer: (a) Both A and R are true, and R is the correct explanation of A.
Case Study Based Question
Case Study:
A student is studying shapes of molecules using VSEPR theory. He makes the following observations:
- In $CH_{4}$, the bond angle is $109.5^{\circ}$.
- In $NH_{3}$, the bond angle decreases to $107^{\circ}$.
- In $H_{2}O$, the bond angle further decreases to $104.5^{\circ}$.
Questions:
(i) Why does the bond angle decrease from $CH_{4}$ to $H_{2}O$?
(ii) Arrange $CH_{4}, NH_{3}, H_{2}O$ in order of increasing repulsion among electron pairs.
(iii) Which molecule has the maximum distortion from ideal tetrahedral geometry?
Answers:
(i) Lone pairs exert stronger repulsion than bond pairs. $CH_{4}$ (no lone pairs), $NH_{3}$ (1 lone pair), $H_{2}O$ (2 lone pairs).
(ii) $CH_{4} < NH_{3} < H_{2}O$
(iii) $H_{2}O$


