Anand Classes explains sp³d², sp³d³, and dsp² Hybridisation with detailed discussion on the geometry and bonding in SF₆, IF₇ molecules, and [Ni(CN)₄]²⁻ ion. Students will learn how the combination of s, p, and d orbitals gives rise to different molecular geometries such as octahedral, pentagonal bipyramidal, and square planar structures. The topic is supported with clear diagrams, step-by-step orbital explanations, MCQs, Q&A, Assertion-Reason, and Case Study questions, making it ideal for Class 11, Class 12, JEE, and NEET chemistry preparation. Click the print button to download study material and notes.
What is sp3d2 Hybridisation?
In sp3d2 Hybridisation, one s, three p and two d-orbitals get hybridised to form six sp3d2-hybrid orbitals, which adopt an octahedral arrangement.
adopt octahedral geometry
How is the Geometry of SF6 Molecule Explained?
The geometry of SF6 molecule can be explained on the basis of sp3d2 hybridisation.
- In SF6, the central sulphur atom has the ground state valence shell configuration is 3s2 3p4.
- To account for the hexavalency in SF6, one electron each from the 3s and 3px orbitals is promoted to 3d orbitals.
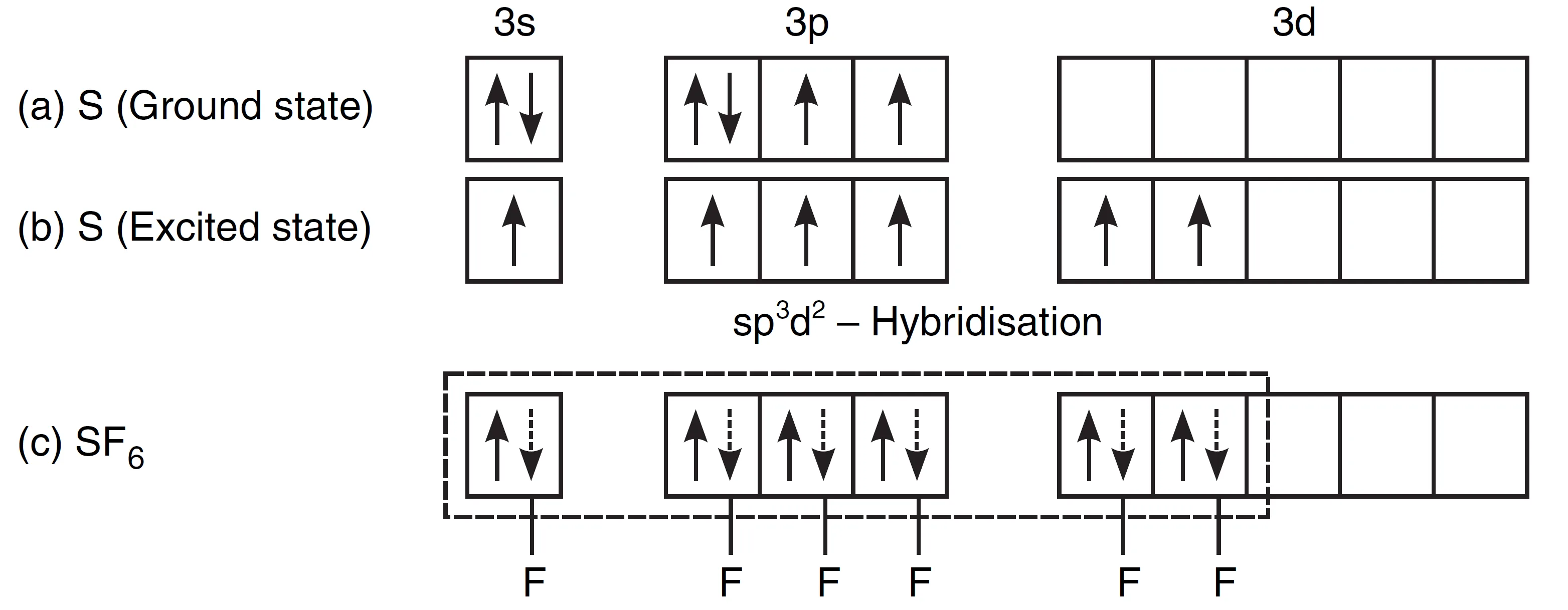
involving sp3d2-hybridisation
- These six orbitals get hybridised to form six sp3d2-hybrid orbitals.
- Each orbital overlaps with the 2p-orbital of fluorine to form an S—F bond.
Thus, the SF6 molecule has an octahedral structure.
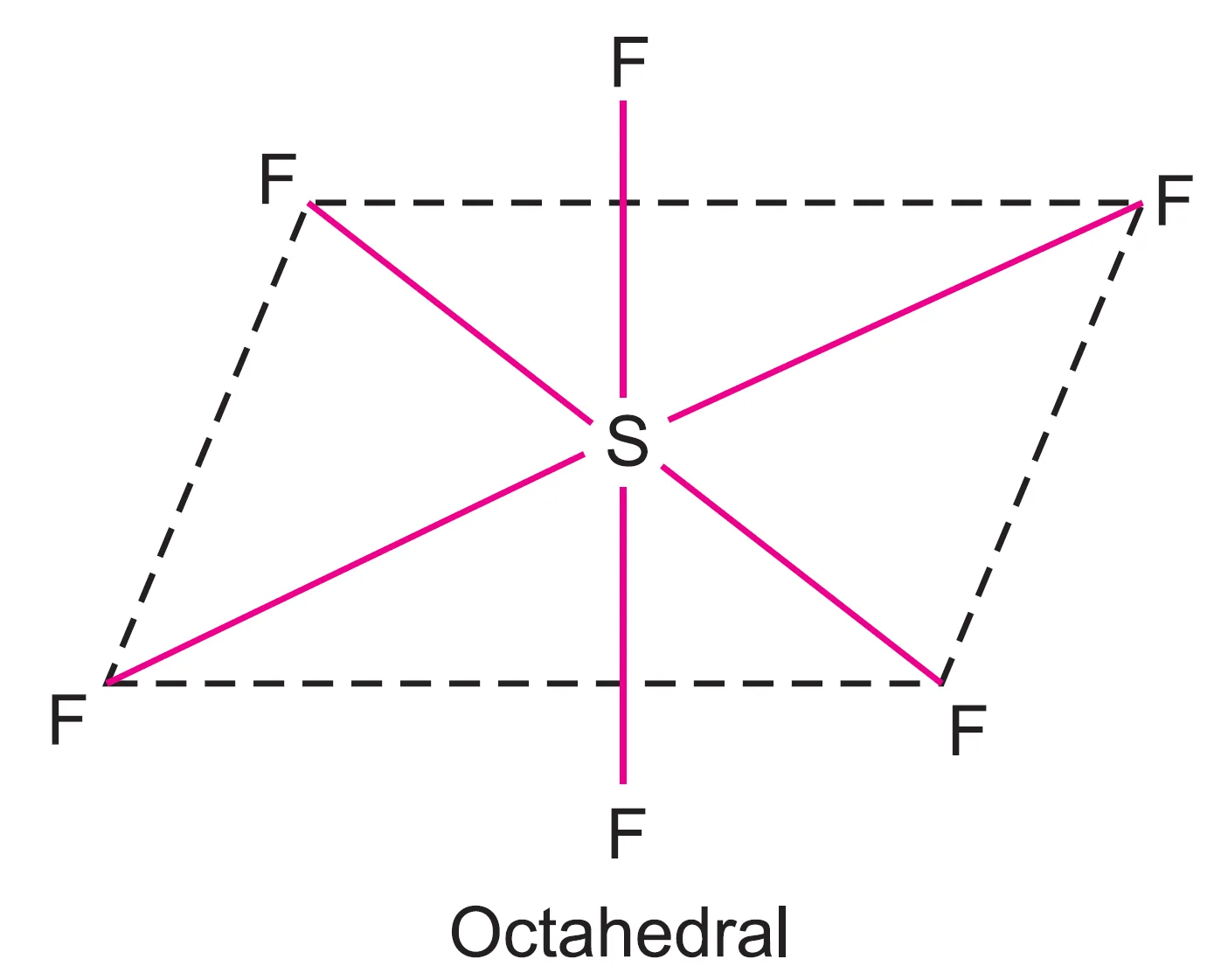
of SF6 molecule.
What is sp3d3 Hybridisation?
This involves the mixing of one s, three p and three d-orbitals, forming seven sp3d3-hybrid orbitals having pentagonal bipyramidal geometry.
How is the Geometry of IF7 Molecule Explained?
The outer electronic configuration of iodine atom is 5s2 5p5.
- To make seven bonds, one s and two p-orbitals are promoted to the higher vacant 5d-orbitals.
- These seven orbitals are then hybridised to give seven sp3d3-hybrid orbitals.
![sp3d2, sp3d3, dsp2 Hybridisation - Geometry of SF6, IF7 Molecules, [Ni(CN)₄]²⁻ Ion-ANAND CLASSES Formation of IF7 molecule
involving sp3d3-hybridisation](https://anandclasses.in/wp-content/uploads/2025/10/image-52.webp)
involving sp3d3-hybridisation
- Each orbital overlaps with 2p-orbitals of fluorine to form the IF7 molecule having pentagonal bipyramidal geometry.
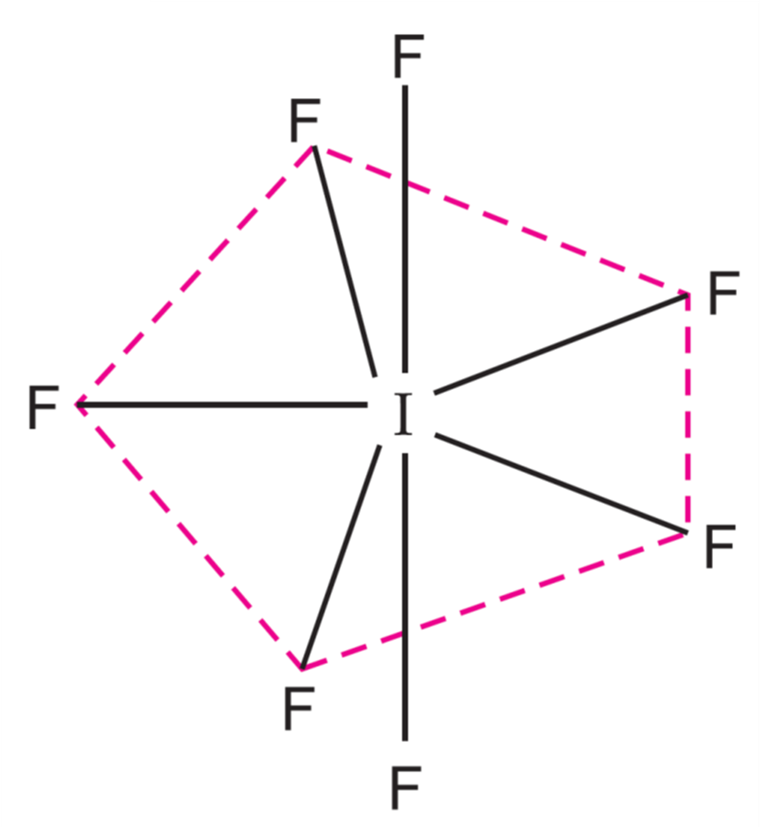
bipyramidal geometry of IF7 molecule
In this geometry:
- Five fluorine atoms are directed towards the vertices of a regular pentagon, making an angle of 72°.
- The other two fluorine atoms are at right angles (90°) to the plane.
- Due to different bond angles, the bonds are of different lengths.
- The axial bonds are longer than equatorial bonds.
What is dsp2 Hybridisation?
In addition to the above types, dsp2-hybridisation is also known, particularly for transition metal ions.
![sp3d2, sp3d3, dsp2 Hybridisation - Geometry of SF6, IF7 Molecules, [Ni(CN)₄]²⁻ Ion-ANAND CLASSES dsp2 Hybridisation](https://anandclasses.in/wp-content/uploads/2025/10/image-53.webp)
- The orbitals involved are $d_{x^2-y^2}$, s and two p orbitals.
- The four orbitals hybridise to give square planar geometry.
How is the Geometry of [Ni(CN)4]2- Ion Explained?
In this case, the oxidation state of Ni is +2.
- The ground state electronic configuration of nickel is $3d^8 4s^2$.
- Therefore, the configuration of Ni2+ is $3d^8$.
- The strong field ligand CN– pairs up the two unpaired d-electrons, leaving one empty 3d orbitals.
- When the four CN– ions come closer to Ni2+ ions, the two unpaired d-electrons are paired up, thereby making one 3d-orbital empty.
- These empty four orbitals (one 3d, one 4s and two 4p) hybridise to form four dsp2-hybrid orbitals.
- Each of the four CN– groups donates a lone pair of electrons to the vacant dsp2-hybrid orbitals.
![Formation of [Ni(CN)4]2– involving dsp2 hybridisation of nickel. The dotted electrons are supplied by CN– ions Formation of [Ni(CN)4]2– involving dsp2 hybridisation of nickel. The dotted electrons are supplied by CN– ions](https://anandclasses.in/wp-content/uploads/2025/10/image-54.webp)
Thus, [Ni(CN)4]2- has a square planar geometry.
![Square planar geometry of Geometry of [Ni(CN)4]2- Ion Square planar geometry of Geometry of [Ni(CN)4]2- Ion](https://anandclasses.in/wp-content/uploads/2025/10/image-55.webp)
It may be noted that in this case, the vacant orbitals are hybridised and the CN– groups donate pairs of electrons into these hybridised orbitals.
🔹 Short Answer Conceptual Questions (SAT)
Q1. What is $\mathrm{sp^3d^2}$ hybridisation?
It is the mixing of one s, three p and two d orbitals to form six equivalent $\mathrm{sp^3d^2}$ orbitals arranged in an octahedral geometry with bond angles of 90°.
Q2. How is $\mathrm{SF_6}$ molecule explained by $\mathrm{sp^3d^2}$ hybridisation?
Sulphur’s electronic configuration:
- Ground state: $\mathrm{3s^2 3p^4}$
- Excited state: $\mathrm{3s^1 3p^3 3d^2}$
These orbitals hybridise to form six $\mathrm{sp^3d^2}$ orbitals, each overlapping with a 2p orbital of F, giving six S–F $\sigma$ bonds in octahedral geometry.
Q3. What is $\mathrm{sp^3d^3}$ hybridisation?
It is the mixing of one s, three p and three d orbitals to form seven equivalent orbitals arranged in pentagonal bipyramidal geometry.
Q4. Explain hybridisation in $\mathrm{IF_7}$.
Iodine:
- Ground state: $\mathrm{5s^2 5p^5}$
- Excited state: $\mathrm{5s^1 5p^3 5d^3}$
These orbitals hybridise into seven $\mathrm{sp^3d^3}$ orbitals.
- Five equatorial orbitals form a pentagon (72^\circ apart)
- Two orbitals are axial (90^\circ to the plane)
Geometry = pentagonal bipyramidal.
Q5. What is $\mathrm{dsp^2}$ hybridisation?
It involves $d_{x^2-y^2}$, s, $p_x$, $p_y$ orbitals, forming four equivalent orbitals with square planar geometry.
Q6. How is $\mathrm{[Ni(CN)_4]^{2-}}$ explained by $\mathrm{dsp^2}$ hybridisation?
- $\mathrm{Ni}$ ground state: $\mathrm{[Ar],3d^8 4s^2}$
- $\mathrm{Ni^{2+}}$: $\mathrm{3d^8}$
Strong field $\mathrm{CN^-}$ ligands cause pairing in 3d orbitals → one 3d orbital becomes vacant.
Hybridisation of $3d, 4s, 4p_x, 4p_y$ → $\mathrm{dsp^2}$ orbitals.
Each accepts lone pairs from CN⁻ → square planar geometry.
🔹 Multiple Choice Questions (MCQs)
Q1. Hybridisation in $\mathrm{SF_6}$ is:
(a) $\mathrm{sp^3}$
(b) $\mathrm{sp^3d}$
(c) $\mathrm{sp^3d^2}$
(d) $\mathrm{dsp^2}$
Answer: (c) $\mathrm{sp^3d^2}$
Explanation: 6 orbitals hybridise → octahedral geometry.
Q2. The geometry of $\mathrm{IF_7}$ is:
(a) Trigonal bipyramidal
(b) Octahedral
(c) Pentagonal bipyramidal
(d) Square planar
Answer: (c) Pentagonal bipyramidal
Explanation: $\mathrm{sp^3d^3}$ → 7 orbitals arranged as 5 equatorial (72° apart) + 2 axial (90°).
Q3. In $\mathrm{[Ni(CN)_4]^{2-}}$, the hybridisation and geometry are:
(a) $\mathrm{sp^3}$, tetrahedral
(b) $\mathrm{dsp^2}$, square planar
(c) $\mathrm{sp^3d}$, trigonal bipyramidal
(d) $\mathrm{sp^3d^2}$, octahedral
Answer: (b) $\mathrm{dsp^2}$, square planar
Explanation: Strong field CN⁻ ligand → $\mathrm{dsp^2}$ hybridisation → square planar.
Q4. In $\mathrm{IF_7}$, bond angles are:
(a) 90° only
(b) 72° and 90°
(c) 120° and 90°
(d) 60° and 90°
Answer: (b) 72° and 90°
Explanation: Equatorial F–I–F = 72°, axial bonds = 90°.
Q5. In $\mathrm{SF_6}$, the number of equivalent S–F bonds is:
(a) 4
(b) 6
(c) 5
(d) 7
Answer: (b) 6
Explanation: Octahedral arrangement makes all six S–F bonds equivalent.
🔹 Assertion–Reason Questions
Q1. Assertion: In $\mathrm{SF_6}$, sulphur undergoes $\mathrm{sp^3d^2}$ hybridisation.
Reason: Six orbitals hybridise into octahedral geometry.
✅ Both true; Reason explains Assertion.
Q2. Assertion: $\mathrm{IF_7}$ has pentagonal bipyramidal geometry.
Reason: Iodine undergoes $\mathrm{sp^3d^3}$ hybridisation.
✅ Both true; Reason explains Assertion.
Q3. Assertion: $\mathrm{[Ni(CN)_4]^{2-}}$ is tetrahedral.
Reason: $\mathrm{dsp^2}$ hybridisation gives four equivalent orbitals.
❌ Assertion false; Reason true. Actual geometry = square planar.
Q4. Assertion: All S–F bonds in $\mathrm{SF_6}$ are equivalent.
Reason: Octahedral geometry arranges bonds symmetrically.
✅ Both true; Reason explains Assertion.
Q5. Assertion: Axial bonds in $\mathrm{IF_7}$ are longer than equatorial bonds.
Reason: Axial bonds experience greater repulsion.
✅ Both true; Reason explains Assertion.
🔹 Case Study
Passage:
Hybridisation involving d orbitals explains hypervalency. In $\mathrm{SF_6}$, sulphur undergoes $\mathrm{sp^3d^2}$ hybridisation giving octahedral geometry with six equivalent bonds. In $\mathrm{IF_7}$, iodine undergoes $\mathrm{sp^3d^3}$ hybridisation, giving pentagonal bipyramidal geometry with 72^\circ and 90^\circ bond angles. In $\mathrm{[Ni(CN)_4]^{2-}}$, $\mathrm{dsp^2}$ hybridisation explains its square planar geometry due to pairing of d-electrons by strong field CN⁻ ligands.
Questions:
- What is the hybridisation of sulphur in $\mathrm{SF_6}$?
→ $\mathrm{sp^3d^2}$ - What is the geometry of $\mathrm{IF_7}$?
→ Pentagonal bipyramidal - What are the bond angles in $\mathrm{IF_7}$?
→ 72° (equatorial) and 90° (axial) - What is the hybridisation of $\mathrm{[Ni(CN)_4]^{2-}}$?
→ $\mathrm{dsp^2}$ - Why is $\mathrm{[Ni(CN)_4]^{2-}}$ square planar?
→ Because CN⁻ ligands pair up d-electrons, allowing $\mathrm{dsp^2}$ hybridisation.
![sp3d2, sp3d3, dsp2 Hybridisation - Geometry of SF6, IF7 Molecules, [Ni(CN)₄]²⁻ Ion-ANAND CLASSES Chemical Bonding And Molecular Structure Class 11 JEE NEET Study Material pdf download free](https://anandclasses.in/wp-content/uploads/2025/09/Chemical-Bonding-And-Molecular-Structure-Class-11-JEE-NEET-Study-Material.webp)

