Anand Classes provides a detailed explanation of sp³d Hybridisation with special reference to the geometry, axial and equatorial bonds in Phosphorus Pentachloride (PCl₅). Students will learn how one s, three p, and one d orbital combine to form five equivalent sp³d hybrid orbitals, resulting in a trigonal bipyramidal structure. The topic is thoroughly explained with diagrams, characteristics, solved examples, MCQs, Q&A, Assertion-Reason, and Case Study questions, making it ideal for Class 11, Class 12, JEE, and NEET preparation. Click the print button to download study material and notes.
What is Hybridisation in Elements Involving d-Orbitals?
The elements of the third period contain d-orbitals in addition to s- and p-orbitals. The 3d-orbitals are comparable in energy to the 3s- and 3p-orbitals. These d-orbitals are also involved in the hybridisation to explain the geometries of molecules of elements of the third period.
- This results in covalencies of 5, 6 and 7, which are not possible in compounds of second-period elements.
- Due to the availability of d-orbitals, phosphorus and sulphur can exhibit covalency of 5 and 6 respectively.
- However, the corresponding elements of the same group, nitrogen and oxygen, cannot extend their octets.
For example:
- In the ground state, phosphorus has three half-filled orbitals and sulphur has two half-filled orbitals (similar to nitrogen and oxygen).
- Thus, they form compounds like PF3 and H2S.
- But P and S also form hypervalent compounds like PF5 and SF6, whereas N and O cannot form NF6 and OF6.
- This is a special feature of third-period elements, as they can extend their octets.
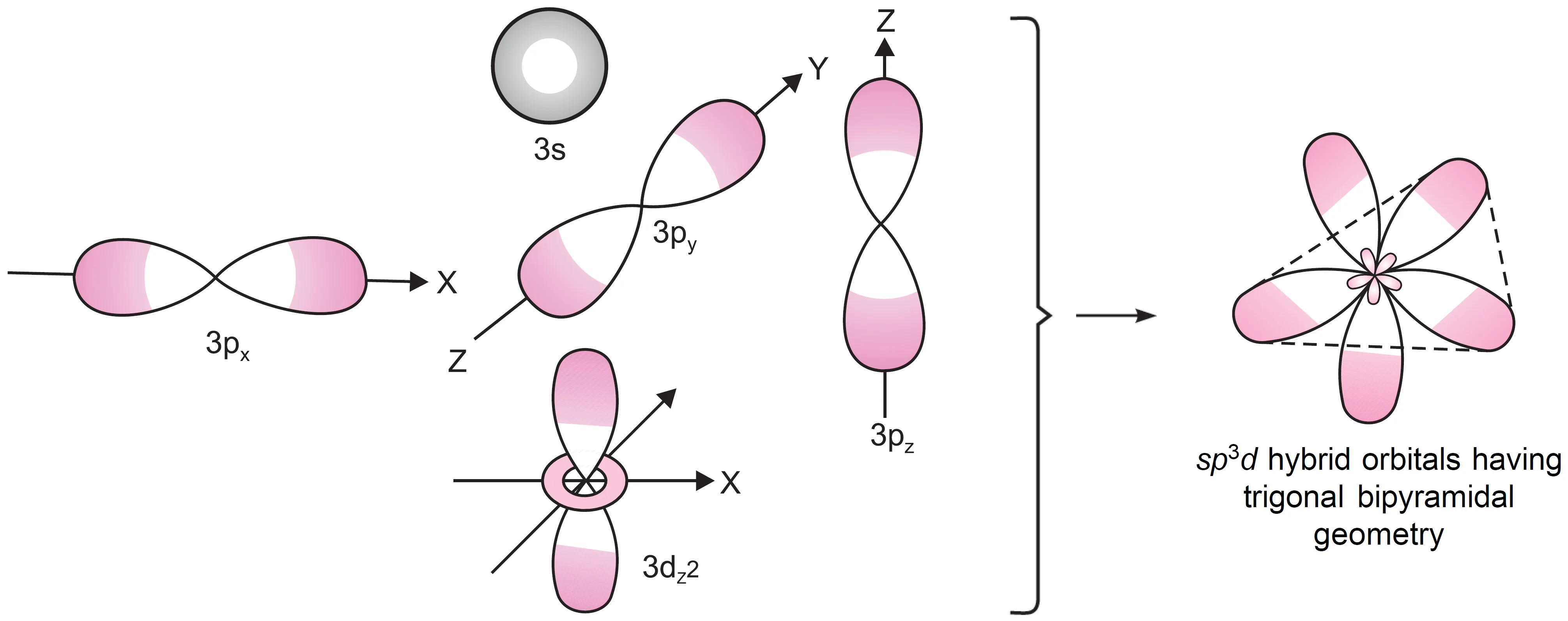
What is sp3d Hybridisation?
This hybridisation involves the mixing of one s, three p, and one d orbital. These five orbitals hybridise to form five sp3d hybrid orbitals.
- These hybrid orbitals point towards the corners of a trigonal bipyramidal geometry.
- In this arrangement:
- Three orbitals form a plane directed towards the corners of an equilateral triangle.
- The other two orbitals are perpendicular to this plane, lying above and below it.
How is the Geometry of PCl5 Molecule Explained by sp3d Hybridisation?
The outer electronic configuration of phosphorus (Z = 15), the central atom, is 3s2 3p3, which means it has three unpaired electrons in the ground state.
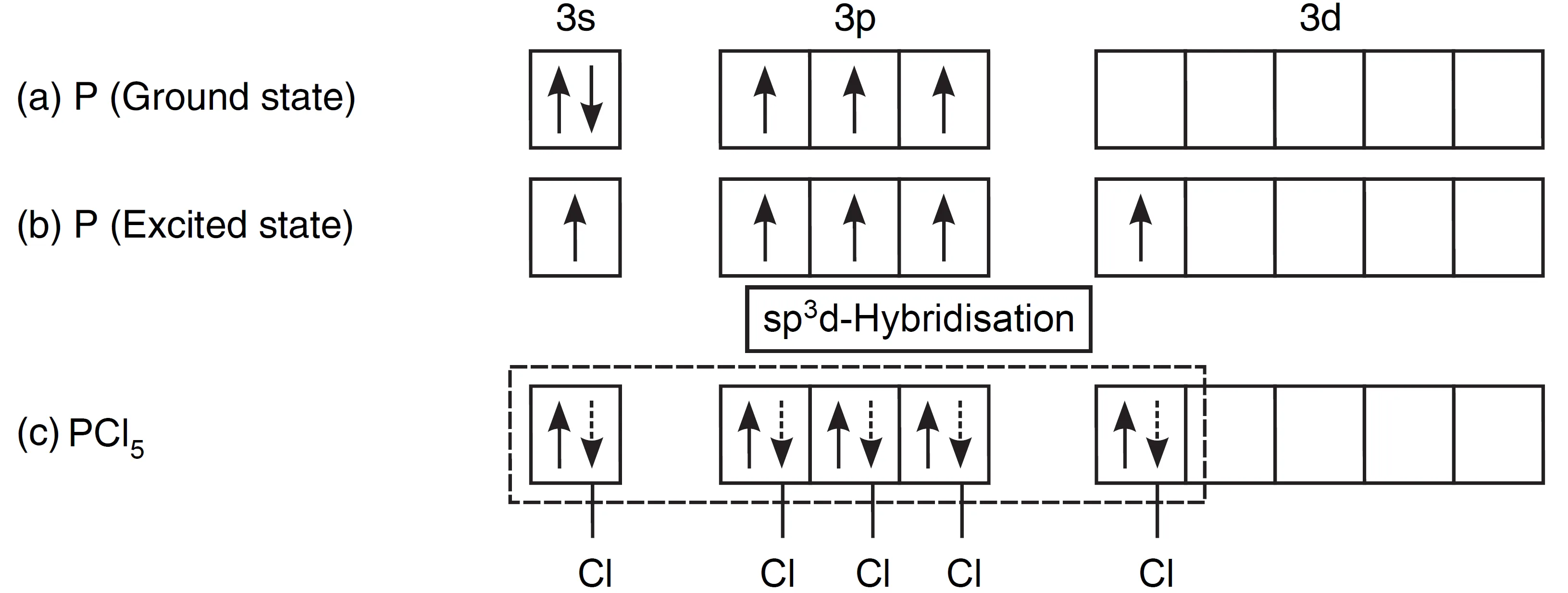
- To explain the pentavalency of phosphorus in PCl5, one electron from the 3s orbital is promoted to the 3d orbital, giving five unpaired electrons (excited state).
- These five orbitals hybridise to form five sp³d orbitals, which adopt a trigonal bipyramidal arrangement.
- Each sp³d orbital overlaps with the 3p orbital of chlorine, forming five P—Cl bonds.
Thus, the PCl5 molecule has trigonal bipyramidal geometry.
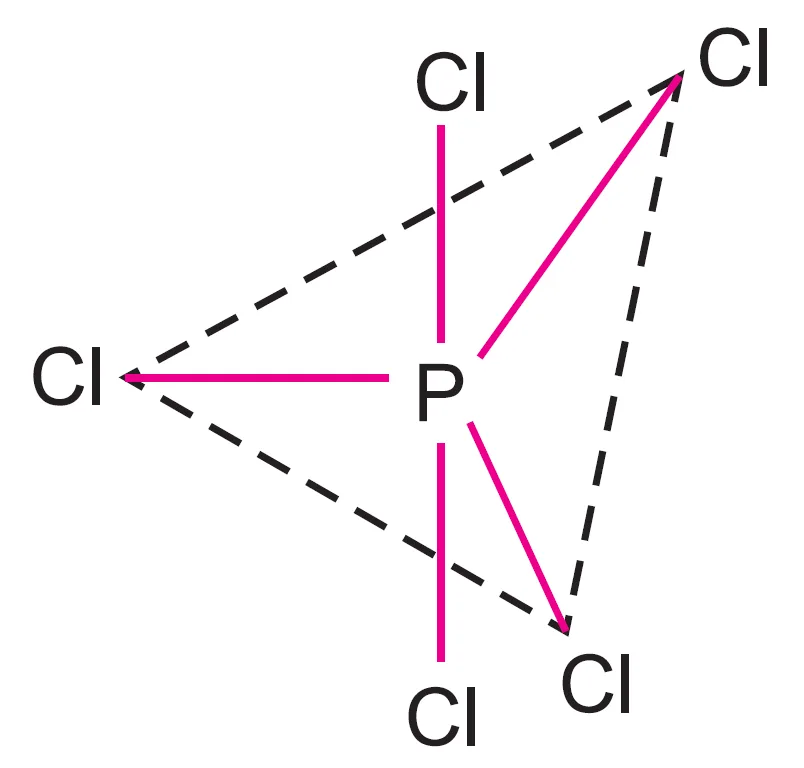
What are Axial and Equatorial Bonds in PCl5?
In the trigonal bipyramidal geometry of PCl5:
- Three bonds lie in one plane at an angle of 120o to one another → called equatorial bonds.
- The other two bonds are perpendicular to this plane, one above and one below, making an angle of 90o → called axial bonds.
It may be noted that this geometry is not symmetrical:
- Axial bonds are slightly longer and weaker than equatorial bonds.
- This is due to greater repulsion from other bonds in axial position (explained by VSEPR theory).
For example, in PCl5:
- Each P—Cl axial bond = 219 pm
- Each P—Cl equatorial bond = 204 pm
Thus, the axial bonds are longer and weaker than equatorial bonds, making PCl5 quite reactive.
Does PF5 Show the Same Hybridisation?
Yes, PF5 also has sp3d hybridisation and adopts the same trigonal bipyramidal geometry as PCl5.
Short Answer Conceptual Type Questions (SAT)
Q1. Why can third-period elements like phosphorus and sulphur form hypervalent compounds while nitrogen and oxygen cannot?
Answer: Third-period elements have vacant 3d orbitals available for hybridisation, allowing them to expand their octet and form compounds such as PF₅ and SF₆. In contrast, nitrogen and oxygen lack d-orbitals, so they cannot exceed the octet rule.
Q2. What is sp³d hybridisation?
Answer: In sp³d hybridisation, one s, three p, and one d orbital mix to form five equivalent sp³d hybrid orbitals, which arrange themselves in a trigonal bipyramidal geometry.
Q3. How does sp³d hybridisation explain the geometry of PCl₅?
Answer: In PCl₅, phosphorus undergoes promotion of one 3s electron to the 3d orbital, resulting in five unpaired electrons. These hybridise to form five sp³d orbitals, which overlap with chlorine 3p orbitals, giving a trigonal bipyramidal structure.
Q4. What are axial and equatorial bonds in PCl₅?
Answer: In trigonal bipyramidal geometry:
- Three equatorial bonds lie in one plane at 120°.
- Two axial bonds lie perpendicular to the equatorial plane at 90°.
The axial bonds are longer and weaker than equatorial ones due to greater repulsion.
Q5. Give bond lengths of axial and equatorial P—Cl bonds in PCl₅.
Answer:
- P—Cl axial bond = 219 pm
- P—Cl equatorial bond = 204 pm
Multiple Choice Questions (MCQs)
Q1. Which of the following molecules shows sp3d hybridisation?
(a) CH₄
(b) BF₃
(c) PCl₅
(d) H₂O
Answer: (c) PCl₅
Explanation: CH₄ → sp³, BF₃ → sp², H₂O → sp³.
Q2. The geometry of sp3d hybridisation is:
(a) Tetrahedral
(b) Trigonal bipyramidal
(c) Square planar
(d) Octahedral
Answer: (b) Trigonal bipyramidal
Q3. In PCl₅, the axial bond length is:
(a) Equal to equatorial bond
(b) Shorter than equatorial bond
(c) Longer than equatorial bond
(d) None of these
Answer: (c) Longer than equatorial bond
Explanation: Axial P—Cl = 219 pm, Equatorial P—Cl = 204 pm.
Q4. Which statement is true for SF₆?
(a) It shows sp³ hybridisation
(b) It shows sp³d² hybridisation
(c) It has octahedral geometry
(d) Both (b) and (c)
Answer: (d) Both (b) and (c)
Q5. Which element cannot form PF₅-type hypervalent compound?
(a) N
(b) P
(c) As
(d) Sb
Answer: (a) N
Explanation: Nitrogen has no vacant d-orbitals to expand its octet.
Assertion–Reason Type Questions
Q1.
Assertion (A): PCl₅ has trigonal bipyramidal geometry.
Reason (R): Phosphorus undergoes sp³d hybridisation.
Answer: Both A and R are true, and R explains A.
Q2.
Assertion (A): The axial bonds in PCl₅ are longer than equatorial bonds.
Reason (R): Axial bonds experience more repulsion from equatorial bonds.
Answer: Both A and R are true, and R explains A.
Q3.
Assertion (A): Nitrogen cannot form NCl₅.
Reason (R): Nitrogen lacks vacant d-orbitals.
Answer: Both A and R are true, and R explains A.
Q4.
Assertion (A): SF₆ has octahedral geometry.
Reason (R): Sulphur undergoes sp³d² hybridisation.
Answer: Both A and R are true, and R explains A.
Case Study
Case Study:
Phosphorus forms both PF₃ and PF₅. In PF₃, phosphorus shows sp³ hybridisation with three bond pairs and one lone pair, giving a pyramidal shape. In PF₅, phosphorus promotes one 3s electron to the 3d orbital, resulting in five unpaired electrons. These hybridise to form five sp³d orbitals, which overlap with fluorine orbitals to form five P—F bonds. The geometry is trigonal bipyramidal with three equatorial bonds (120°) and two axial bonds (90° to the plane). Axial bonds are longer and weaker than equatorial bonds, making PF₅ reactive.
Questions:
- What is the difference between hybridisation in PF₃ and PF₅?
- Why is PF₅ trigonal bipyramidal?
- Which bonds in PF₅ are weaker, axial or equatorial?
- Why can phosphorus form PF₅ but nitrogen cannot form NF₅?
- What are the bond angles in trigonal bipyramidal geometry?
Answers:
- PF₃ → sp³ (pyramidal), PF₅ → sp³d (trigonal bipyramidal).
- Due to sp³d hybridisation.
- Axial bonds (longer and weaker).
- Nitrogen lacks d-orbitals.
- Equatorial = 120°, Axial–Equatorial = 90°.


