Anand Classes explains sp Hybridisation in detail through the formation of Beryllium Fluoride (BeF₂) and Acetylene (C₂H₂) molecules. Students will understand how one s orbital and one p orbital combine to form two equivalent sp hybrid orbitals that lie linearly at 180°, giving rise to linear molecular geometry. The concept is supported with diagrams, solved examples, MCQs, Q&A, Assertion-Reason, and Case Study questions, making it perfect for Class 11, Class 12, JEE, and NEET chemistry preparation. Click the print button to download study material and notes.
What is sp Hybridisation?
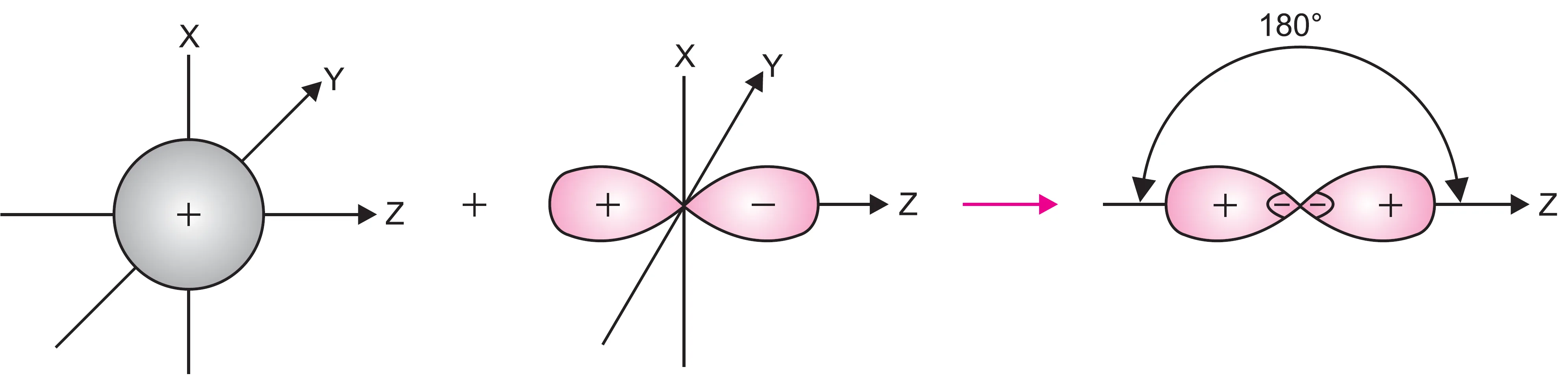
In sp hybridisation, one s and one p orbital mix to form two equivalent sp hybrid orbitals.
- The orbitals suitable for sp hybridisation are s and pz orbitals if the hybrid orbitals are to lie along the Z–axis.
- Each sp hybrid orbital has 50% s–character and 50% p–character.
- These two sp hybrid orbitals point in opposite directions along the Z-axis with bigger positive lobes and very small negative lobes.
- This provides for more effective overlap resulting in the formation of stronger bonds.
- These two sp hybrid orbitals lie along a linear arrangement, therefore sp hybridisation is also called linear hybridisation.
How is the Shape of BeF2 Molecule Explained by sp Hybridisation?
The electronic configuration of beryllium is 1s2 2s2.
- To account for divalency of Be, one of the 2s electrons is promoted to the vacant 2p orbital.
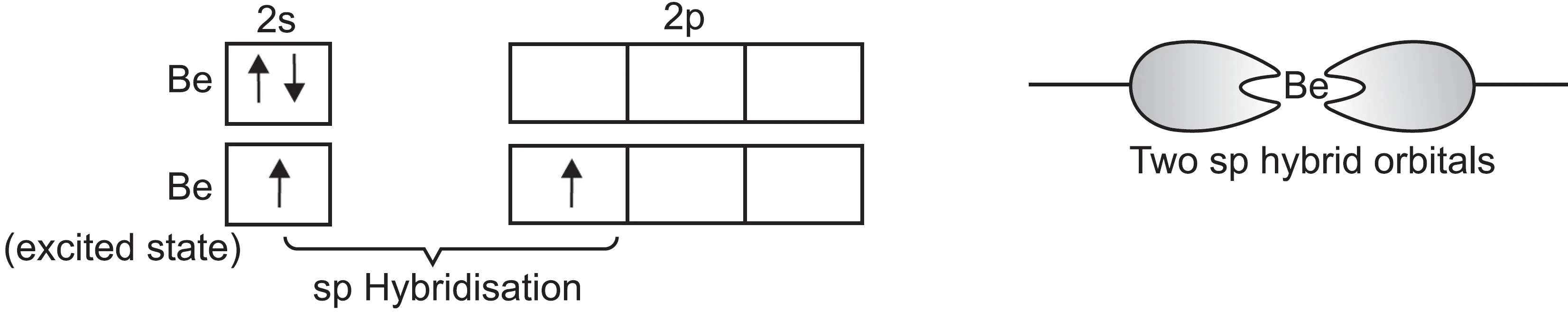
- These two orbitals (2s and 2p) hybridise to form two sp hybrid orbitals.
- These sp hybrid orbitals are oriented in a linear arrangement (180o).
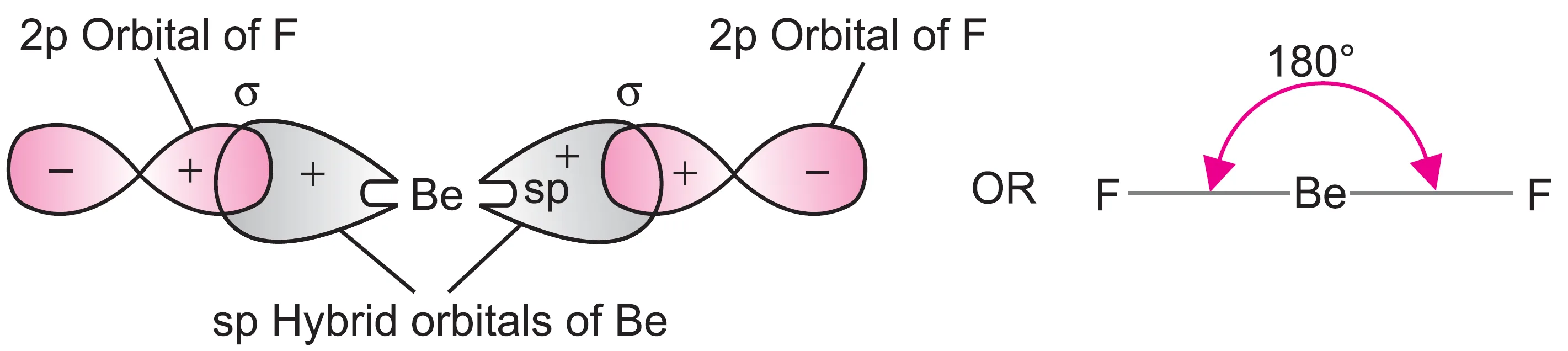
- Each sp hybrid orbital overlaps with a 2p orbital of fluorine to form two Be—F σ bonds.
- Thus, BeF2 is linear and has a bond angle of 180p.
How Does Carbon Undergo sp Hybridisation?
In sp hybridisation of carbon:
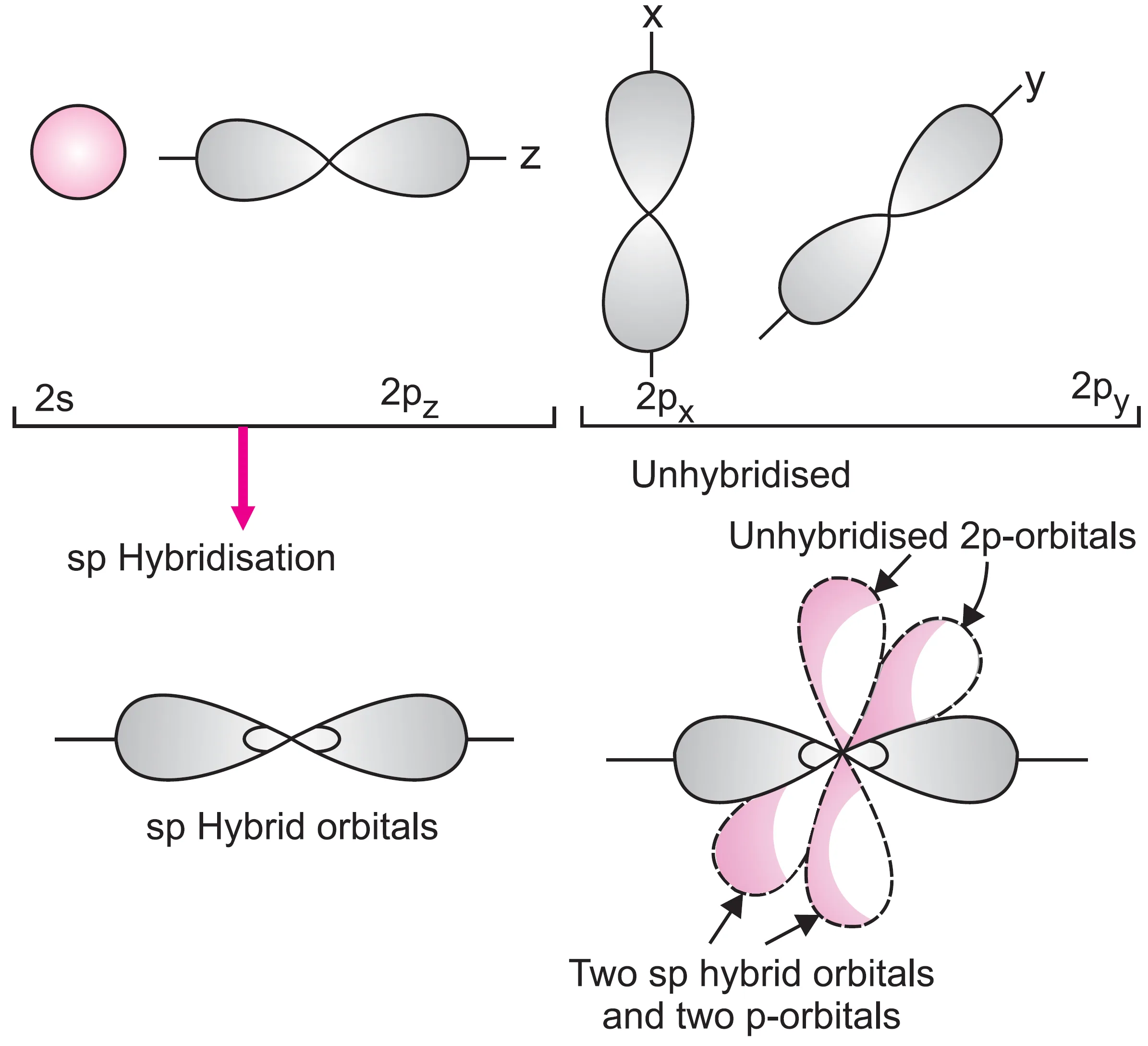
- One s orbital (2s) and one p orbital (2px) hybridise in the valence shell of the excited carbon atom.
- This forms two equivalent sp orbitals, while the remaining two orbitals (2py and 2pz) do not take part in hybridisation.
- Due to mutual repulsion of electron clouds, the two sp orbitals are oriented at an angle of 180o.
- Hence, sp hybridisation is also called diagonal hybridisation.
- Each sp orbital has equal s- and p-character (50% each).
- The two unhybridised orbitals are directed along the y-axis and z-axis, while the two hybridised orbitals are directed along the x-axis.
What is an Example of sp Hybridisation in Acetylene (C2H2)?
The molecule acetylene (ethyne, HC ≡ CH) illustrates sp hybridisation.
- Each carbon atom undergoes sp hybridisation, leaving two unhybridised orbitals (2py and 2pz).
- One sp orbital of each carbon overlaps axially with the sp orbital of the other carbon to form a C—C σ bond.
- The remaining sp orbital of each carbon overlaps with the 1s orbital of hydrogen to form two C—H σ bonds.
- Each of the two unhybridised orbitals (2py and 2pz) of one carbon overlaps sidewise with the corresponding orbitals of the other carbon to form two π bonds.
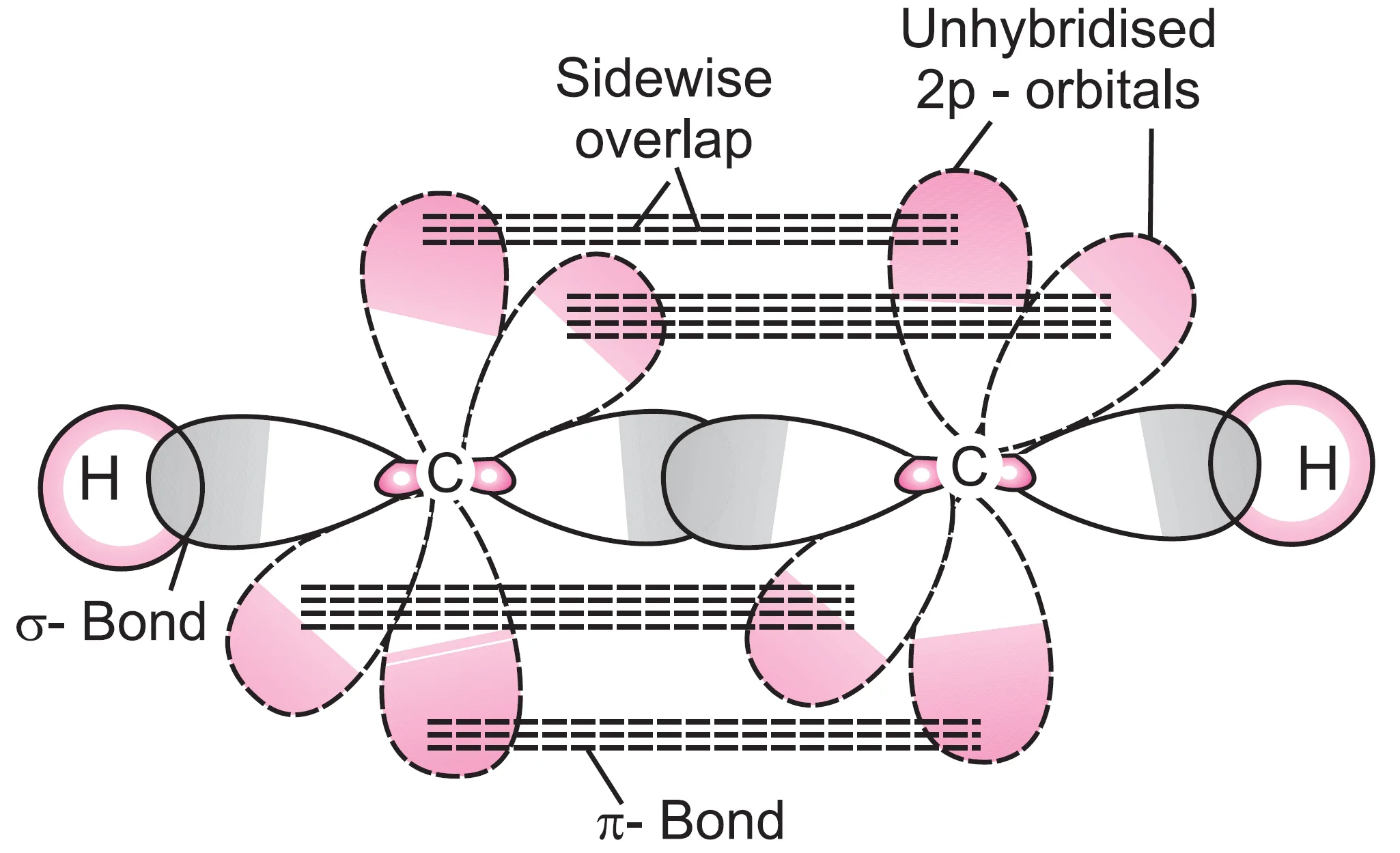
Thus, the C ≡ C triple bond consists of one σ bond and two π bonds.
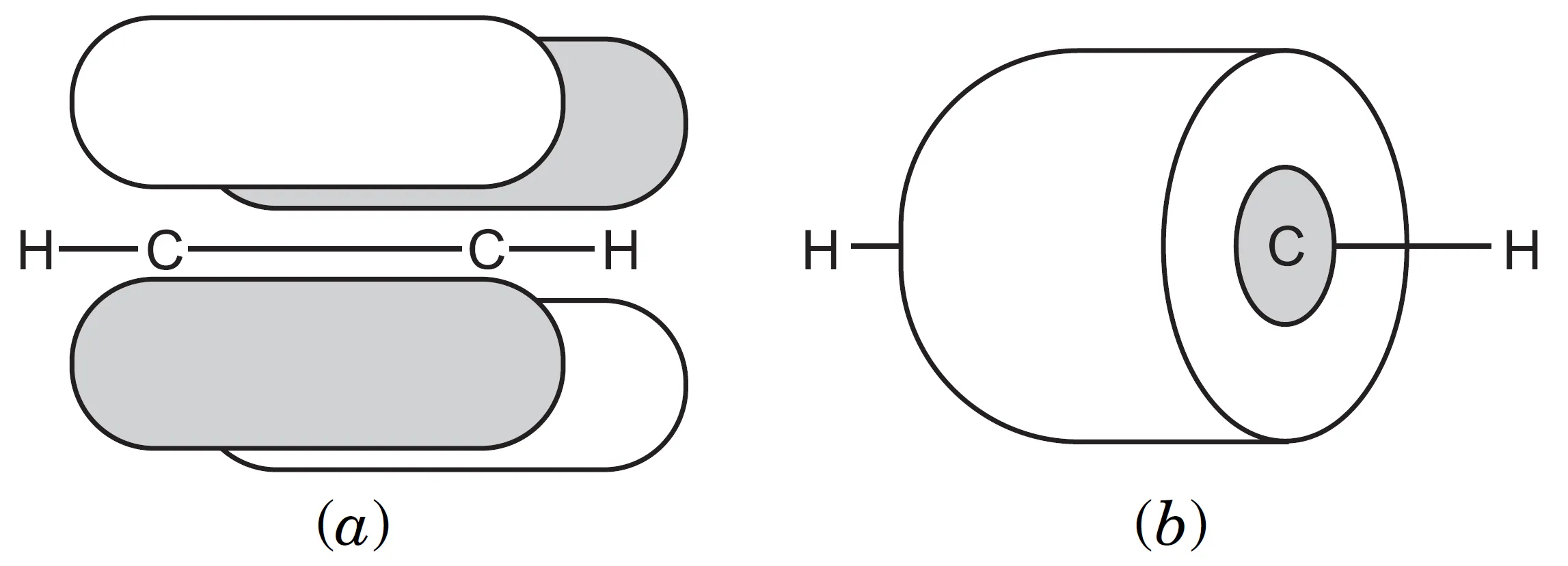
- If one π cloud lies above and below the internuclear axis, the other π cloud lies in front and behind the internuclear axis.
- The overlapping π clouds merge to form a single cylindrical electron cloud around the internuclear axis.
In acetylene (HC ≡ CH):
- C ≡ C bond length = 120 pm
- C—H bond length = 106 pm
- H—C—C bond angle = 180o
Short Answer Conceptual Type Questions (SAT)
Q1. What is sp hybridisation?
Answer: In sp hybridisation, one $s$ orbital and one $p$ orbital mix to form two equivalent sp hybrid orbitals. Each orbital has 50% $s$-character and 50% $p$-character and are oriented in a linear geometry with a bond angle of $180^\circ$.
Q2. Why is sp hybridisation also called linear hybridisation?
Answer: Because the two sp hybrid orbitals are oriented in opposite directions (along the same axis), giving a linear arrangement with a bond angle of $180^\circ$.
Q3. How does sp hybridisation occur in BeF2?
Answer: In $\mathrm{BeF_2}$, beryllium ($1s^2 ; 2s^2$) promotes one $2s$ electron to $2p$, and the orbitals ($2s$ and $2p$) hybridise to form two sp orbitals. Each overlaps with a fluorine 2p orbital, forming two Be—F $\sigma$ bonds. The molecule is linear with a bond angle of $180^\circ$.
Q4. How does carbon undergo sp hybridisation in acetylene ($\mathrm{C_2H_2}$)?
Answer: In acetylene, each carbon undergoes sp hybridisation, forming two sp orbitals and leaving two unhybridised $p$ orbitals ($2p_y, 2p_z$). One sp orbital overlaps with another carbon sp orbital (C—C $sigma$ bond), the other overlaps with hydrogen ($mathrm{C—H}$ $sigma$ bond). The unhybridised $p$ orbitals overlap sidewise to form two $\pi$ bonds, giving a C≡C triple bond.
Q5. What is the bond structure of the C≡C bond in acetylene?
Answer: The C≡C bond consists of one $\sigma$ bond and two $\pi$ bonds.
Multiple Choice Questions (MCQs)
Q1. In sp hybridisation, the geometry of the molecule is:
(a) Trigonal planar
(b) Linear
(c) Tetrahedral
(d) Bent
Answer: (b) Linear
Explanation: Two sp orbitals point in opposite directions along the axis, giving $180^\circ$ bond angle.
Q2. The $s$-character in sp hybrid orbitals is:
(a) 25%
(b) 33.3%
(c) 50%
(d) 75%
Answer: (c) 50%
Explanation: sp orbital = $1s + 1p$ → $1/2 = 50%$ s-character.
Q3. Which of the following molecules shows sp hybridisation?
(a) $\mathrm{BeCl_2}$
(b) $\mathrm{CH_4}$
(c) $\mathrm{BF_3}$
(d) $\mathrm{NH_3}$
Answer: (a) $\mathrm{BeCl_2}$
Explanation: $\mathrm{BeCl_2}$ → sp, $\mathrm{CH_4}$ → sp³, $\mathrm{BF_3}$ → sp², $\mathrm{NH_3}$ → sp³.
Q4. In acetylene ($\mathrm{C_2H_2}$), the total number of $\sigma$ and $\pi$ bonds is:
(a) 2 $\sigma$, 2 $\pi$
(b) 3 $\sigma$, 2 $\pi$
(c) 2 $\sigma$, 3 $\pi$
(d) 3 $\sigma$, 1 $\pi$
Answer: (b) 3 $\sigma$, 2 $\pi$
Explanation: In $\mathrm{C_2H_2}$ → 1 C—C $\sigma$, 2 C—H $\sigma$, 2 C—C $\pi$.
Q5. The bond angle in $\mathrm{BeF_2}$ is:
(a) $104.5^\circ$
(b) $107^\circ$
(c) $109.5^\circ$
(d) $180^\circ$
Answer: (d) $180^\circ$
Explanation: $\mathrm{BeF_2}$ has linear geometry due to sp hybridisation.
Assertion–Reason Type Questions
Q1.
Assertion (A): In sp hybridisation, the two hybrid orbitals are oriented at $180^\circ$.
Reason (R): This minimises repulsion between the electron clouds.
Answer: Both A and R are true, and R explains A.
Q2.
Assertion (A): Acetylene has a C≡C triple bond.
Reason (R): Each carbon atom is sp hybridised and forms one $\sigma$ and two $\pi$ bonds.
Answer: Both A and R are true, and R explains A.
Q3.
Assertion (A): $\mathrm{BeF_2}$ is a linear molecule.
Reason (R): Beryllium is sp hybridised, forming two sp orbitals at $180^\circ$.
Answer: Both A and R are true, and R explains A.
Q4.
Assertion (A): A $\pi$ bond is weaker than a $\sigma$ bond.
Reason (R): A $\pi$ bond is formed by sidewise overlap of orbitals, whereas a $\sigma$ bond is formed by head-on overlap.
Answer: Both A and R are true, and R explains A.
Case Study
Case Study:
Acetylene ($\mathrm{C_2H_2}$) is the simplest alkyne, showing sp hybridisation. Each carbon atom undergoes sp hybridisation, forming two sp orbitals directed linearly at $180^\circ$. One sp orbital of each carbon overlaps to form a C—C $\sigma$ bond, while the other overlaps with hydrogen to form C—H $\sigma$ bonds. The unhybridised $p$ orbitals ($2p_y, 2p_z$) on each carbon overlap sidewise to form two $\pi$ bonds, giving rise to a C≡C triple bond.
Experimental values:
- C≡C bond length = 120 pm
- C—H bond length = 106 pm
- H—C—C bond angle = $180^\circ$
The two $\pi$ bonds produce electron clouds that merge to form a cylindrical distribution of electron density around the internuclear axis, explaining the strength and geometry of the triple bond.
Questions:
- What is the $s$-character in sp hybrid orbitals?
- How many $\sigma$ and $\pi$ bonds are present in acetylene?
- Why is the bond angle in $\mathrm{BeF_2}$ equal to $180^\circ$?
- Why is a $\pi$ bond weaker than a $\sigma$ bond?
- What is the bond length of C≡C in acetylene?
Answers:
- $50%$ $s$-character
- 3 $\sigma$ bonds and 2 $\pi$ bonds
- Because sp hybrid orbitals orient linearly at $180^\circ$
- Because sidewise overlap is less effective than head-on overlap
- 120 pm


