Anand Classes presents detailed Class 11 Chemistry notes on VSEPR Theory – Shapes of Molecules (Molecular Geometry), Bond Angles, and Examples to help you understand how electron pair repulsion determines the 3D arrangement of atoms in a molecule. Learn the geometry of molecules like BeCl2 (linear), BF3 (trigonal planar), CH4 (tetrahedral), NH3 (trigonal pyramidal), and H2O (bent), along with bond angle variations due to lone pairs. These notes include diagrams, solved examples, MCQs, and Q&A for NEET, JEE, and CBSE exams. Click the print button to download study material and notes.
How are Shapes of Molecules Explained on the Basis of VSEPR Theory?
This theory is very simple to use. In this theory, no distinction is made between s- and p-electrons. We take into account only the number of electron pairs present in the valence shell of the central atom.
Thus, the particular geometry of a molecule depends upon the number of electron pairs around the central atom.
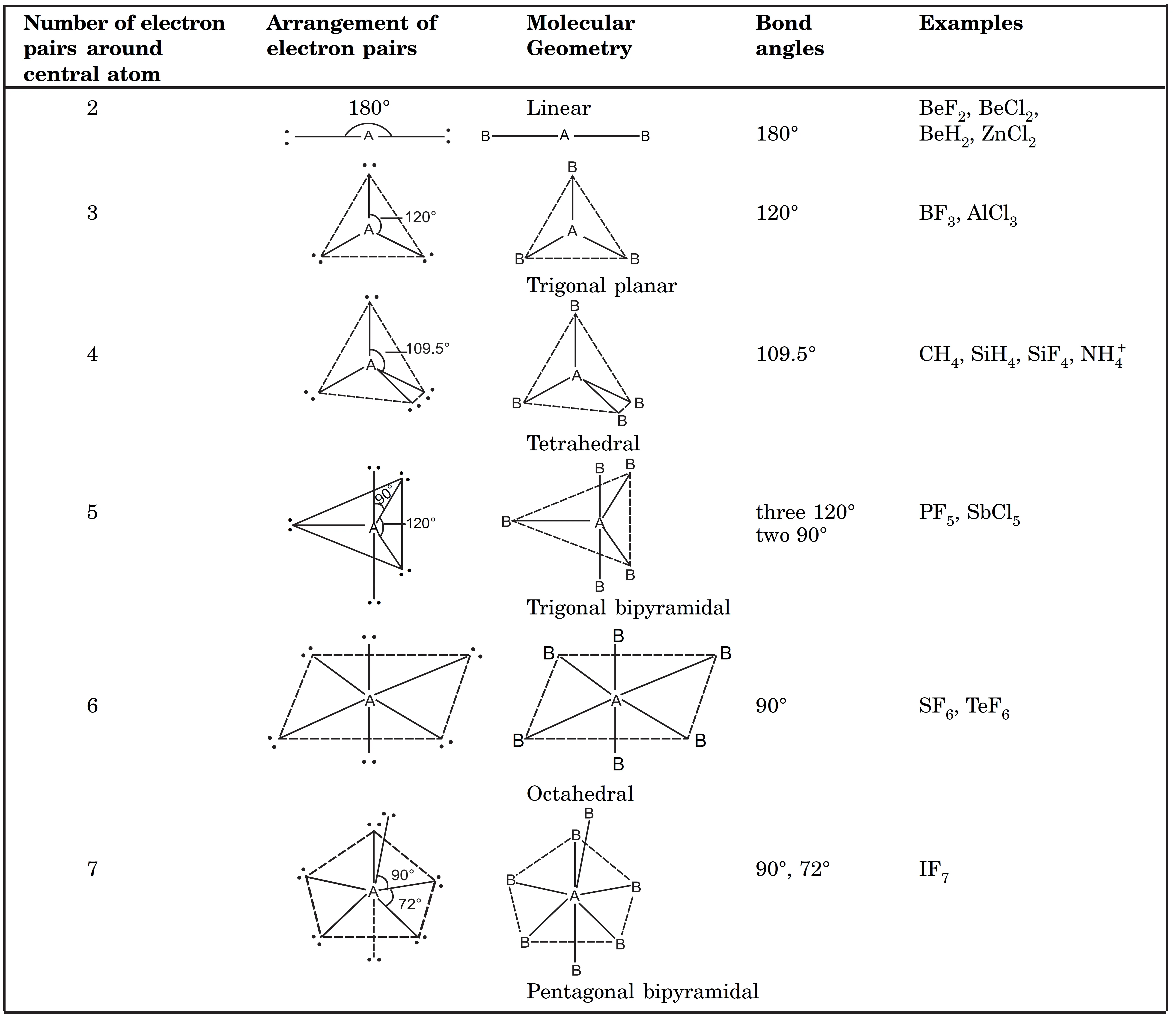
- If there are two electron pairs around the central atom, the only way to keep them as far apart as possible is to arrange them at an angle of 180o to each other. The molecule in such a case will adopt linear geometry.
- For three electron pairs around the central atom, the molecule adopts trigonal planar geometry.
- For four electron pairs around the central atom, the molecule adopts tetrahedral geometry.
- The molecules having five electron pairs around the central atom adopt trigonal bipyramidal geometry.
- The molecules having six electron pairs around the central atom adopt octahedral geometry.
What are the Shapes of Molecules According to VSEPR Theory?
Following Table shows the Geometries of molecules on the basis of VSEPR theory.
| Number of electron pairs around central atom | Arrangement of electron pairs | Molecular Geometry | Bond Angles | Examples |
|---|---|---|---|---|
| 2 | Linear | $180^{\circ}$ | $BeF_{2}$, $BeCl_{2}$, $BeH_{2}$, $ZnCl_{2}$ | |
| 3 | Trigonal planar | $120^{\circ}$ | $BF_{3}$, $AlCl_{3}$ | |
| 4 | Tetrahedral | $109.5^{\circ}$ | $CH_{4}$, $SiH_{4}$, $SiF_{4}$, $NH_{4}^{+}$ | |
| 5 | Three at $120^{\circ}$, two at $90^{\circ}$ | Trigonal bipyramidal | $120^{\circ}$, $90^{\circ}$ | $PF_{5}$, $SbCl_{5}$ |
| 6 | Octahedral | $90^{\circ}$ | $SF_{6}$, $TeF_{6}$ | |
| 7 | Five at $72^{\circ}$, two at $90^{\circ}$ | Pentagonal bipyramidal | $72^{\circ}$, $90^{\circ}$ | $IF_{7}$ |
Short Answer Conceptual Type Questions (SAT) on Molecular Geometry
Q1. Why does BeF2 have a linear geometry?
Answer: BeF2 has two electron pairs around the central atom. To minimize repulsion, they are arranged 180o apart, giving a linear geometry.
Q2. What is the bond angle in BF3 and why?
Answer: BF3 has three bond pairs around boron. They repel each other equally and arrange themselves at 120o, giving a trigonal planar geometry.
Q3. Why does CH4 have a tetrahedral shape?
Answer: Carbon has four bond pairs with hydrogen. To minimize repulsion, these pairs adopt a tetrahedral arrangement with bond angles of 109.5o.
Q4. Explain why PF5 is trigonal bipyramidal.
Answer: In PF5, phosphorus has five bond pairs. Three bond pairs lie in one plane at 120o (equatorial), and two lie perpendicular to this plane at 90o (axial). Hence, the shape is trigonal bipyramidal.
Q5. Give the geometry and bond angle of SF6.
Answer: SF6 has six bond pairs around sulfur. They are arranged symmetrically at 90o, giving an octahedral geometry.
Q6. Why does IF7 adopt a pentagonal bipyramidal structure?
Answer: IF7 has seven bond pairs around iodine. Five lie in a plane forming a pentagon (72o apart), and two occupy positions above and below the plane at 90o.
Multiple Choice Questions (MCQs) on Molecular Geometry
Q1. The bond angle in CH4 is:
(a) 90o
(b) 109.5o
(c) 120o
(d) 180o
Answer: (b) 109.5o
Explanation: Four bond pairs form a tetrahedral geometry with bond angle 109.5o.
Q2. Which of the following molecules is trigonal planar?
(a) NH3
(b) BF3
(c) H3O
(d) CH3
Answer: (b) BF3
Explanation: BF3 has three bond pairs and no lone pair, so geometry is trigonal planar.
Q3. In PF5, the equatorial bond angle is:
(a) 90o
(b) 72o
(c) 120o
(d) 180o
Answer: (c) 120o
Explanation: In trigonal bipyramidal geometry, equatorial bonds are at 120o.
Q4. The geometry of SF6 is:
(a) Tetrahedral
(b) Trigonal planar
(c) Octahedral
(d) Pentagonal bipyramidal
Answer: (c) Octahedral
Explanation: Six bond pairs around sulfur lead to octahedral geometry.
Q5. Which molecule has bond angles of both 72o and 90o ?
(a) BeF2
(b) PF5
(c) IF7
(d) SF6
Answer: (c) IF7
Explanation: Pentagonal bipyramidal structure has five bonds in a pentagon (72o) and two axial bonds at 90o.
Assertion–Reason Questions on Molecular Geometry
Q1.
Assertion (A): BF3 is planar with bond angles of 120o.
Reason (R): Boron in BF3 has three bond pairs and no lone pairs.
Answer: Both A and R are true, and R is the correct explanation of A.
Q2.
Assertion (A): CH4 has bond angles of 109.5o.
Reason (R): The four bond pairs of CH4 repel equally and orient themselves in a tetrahedral arrangement.
Answer: Both A and R are true, and R is the correct explanation of A.
Q3.
Assertion (A): In SF6, all bond angles are 90o.
Reason (R): SF6 adopts an octahedral geometry.
Answer: Both A and R are true, and R is the correct explanation of A.
Q4.
Assertion (A): In PF5, all bond angles are equal.
Reason (R): PF5 has five bonds around phosphorus.
Answer: A is false, R is true.
Explanation: In PF5, there are two types of bond angles — 90o (axial-equatorial) and 120o (equatorial-equatorial).
Case Study Based Questions on Molecular Geometry
Passage:
The shape of molecules can be predicted using VSEPR theory, which depends on the number of electron pairs around the central atom. Molecules like $BeF_{2}$, $BF_{3}$, $CH_{4}$, $PF_{5}$, $SF_{6}$, and $IF_{7}$ adopt linear, trigonal planar, tetrahedral, trigonal bipyramidal, octahedral, and pentagonal bipyramidal geometries, respectively. Bond angles vary accordingly to minimize repulsion between electron pairs.
Questions:
Q1. What is the bond angle in $BeF_{2}$?
Answer: $180^{\circ}$ (linear geometry).
Q2. Why is $CH_{4}$ not planar but tetrahedral?
Answer: Four bond pairs repel each other equally and orient at $109.5^{\circ}$ in 3D space to minimize repulsion.
Q3. Which molecule among $PF_{5}$, $SF_{6}$, and $IF_{7}$ has the highest coordination number?
Answer: $IF_{7}$ (coordination number 7).
Q4. Why does $SF_{6}$ adopt octahedral geometry instead of trigonal bipyramidal?
Answer: $SF_{6}$ has six bond pairs, which minimize repulsion by arranging symmetrically at $90^{\circ}$ in an octahedral structure.


