Anand Classes provides detailed NCERT Solutions for Class 11 Chemistry Chapter – Chemical Bonding and Molecular Structure (Questions 4.1 , 4.2, 4.3, 4.4, 4.5, 4.6, 4.7, 4.8, 4.9, 4.10) with step-by-step explanations, formulas, and concept clarity. These solutions help students understand the fundamental concepts of ionic bond, covalent bond, coordinate bond, hybridisation, molecular orbital theory, and VSEPR theory. Each NCERT question is solved according to the latest CBSE guidelines and exam patterns, making it extremely useful for Class 11 Board Exams, JEE, and NEET Chemistry preparation. The answers are easy to understand and include reasoning, diagrams, and important definitions from the NCERT textbook. Click the print button to download study material and notes.
Explain the formation of a chemical bond
Answer:
The atoms of different elements combine with each other in order to complete their octet (8 electrons) or duplet (2 electrons) in the valence shell, thereby attaining the nearest noble gas configuration.
Key Takeaways:
- Bond formation happens to achieve stability.
- Atoms combine to achieve octet or duplet configuration.
Write Lewis dot symbols for atoms of the elements Mg, Na, B, O, N, and Br
Answer:
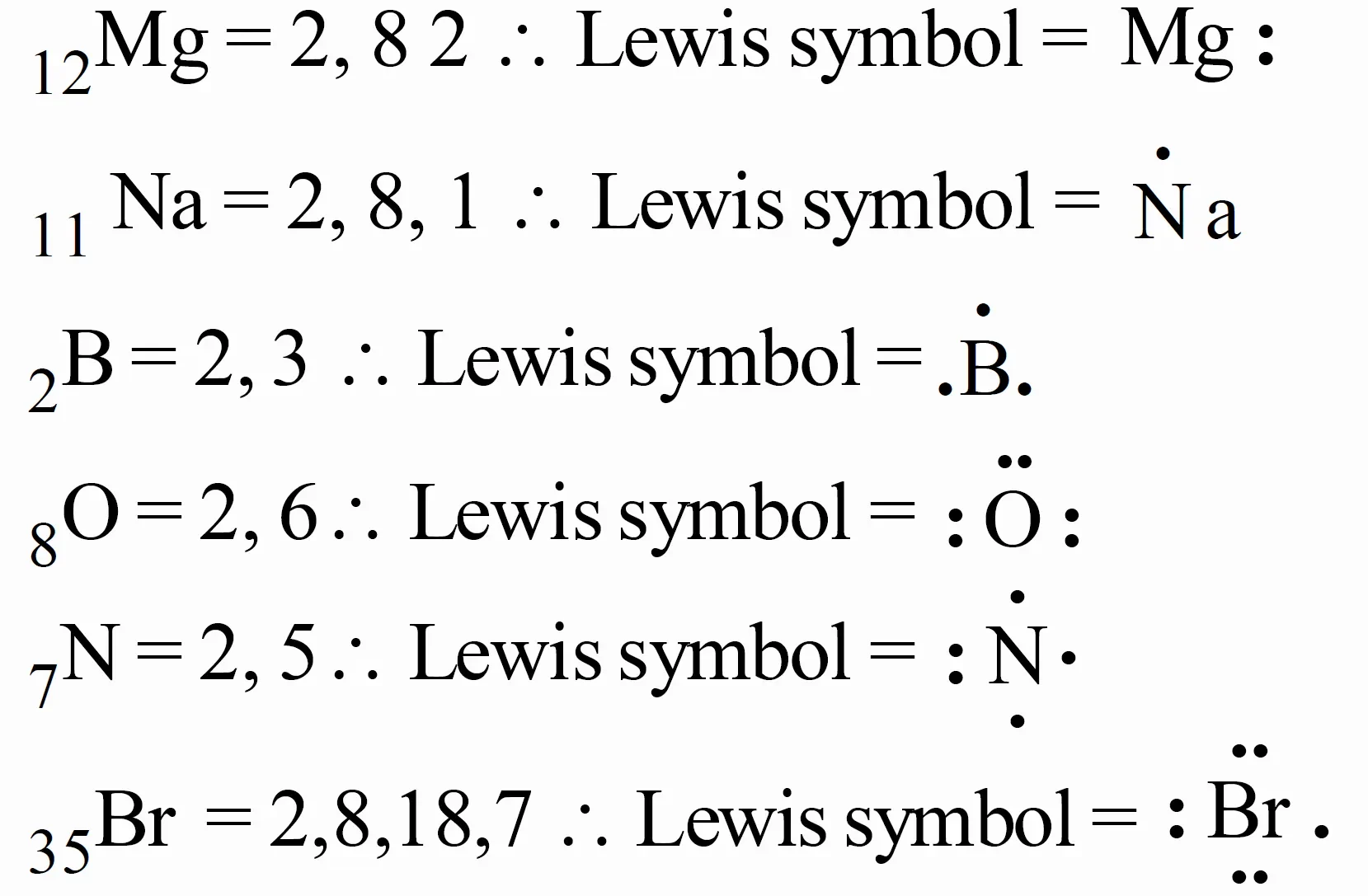
Write Lewis Symbols for following Atoms and Ions S and S2-⁻, Al and Al3+, H and H–
Answer:
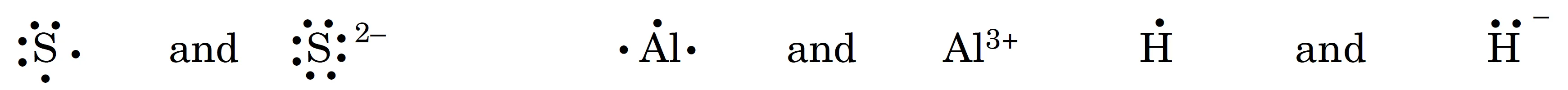
Key Takeaways:
- Cations lose electrons → fewer/no valence electrons.
- Anions gain electrons → more valence electrons.
Draw the Lewis Structures for the following Molecules and Ions H2S, SiCl4, BeF2, CO32- and HCOOH
Answer:
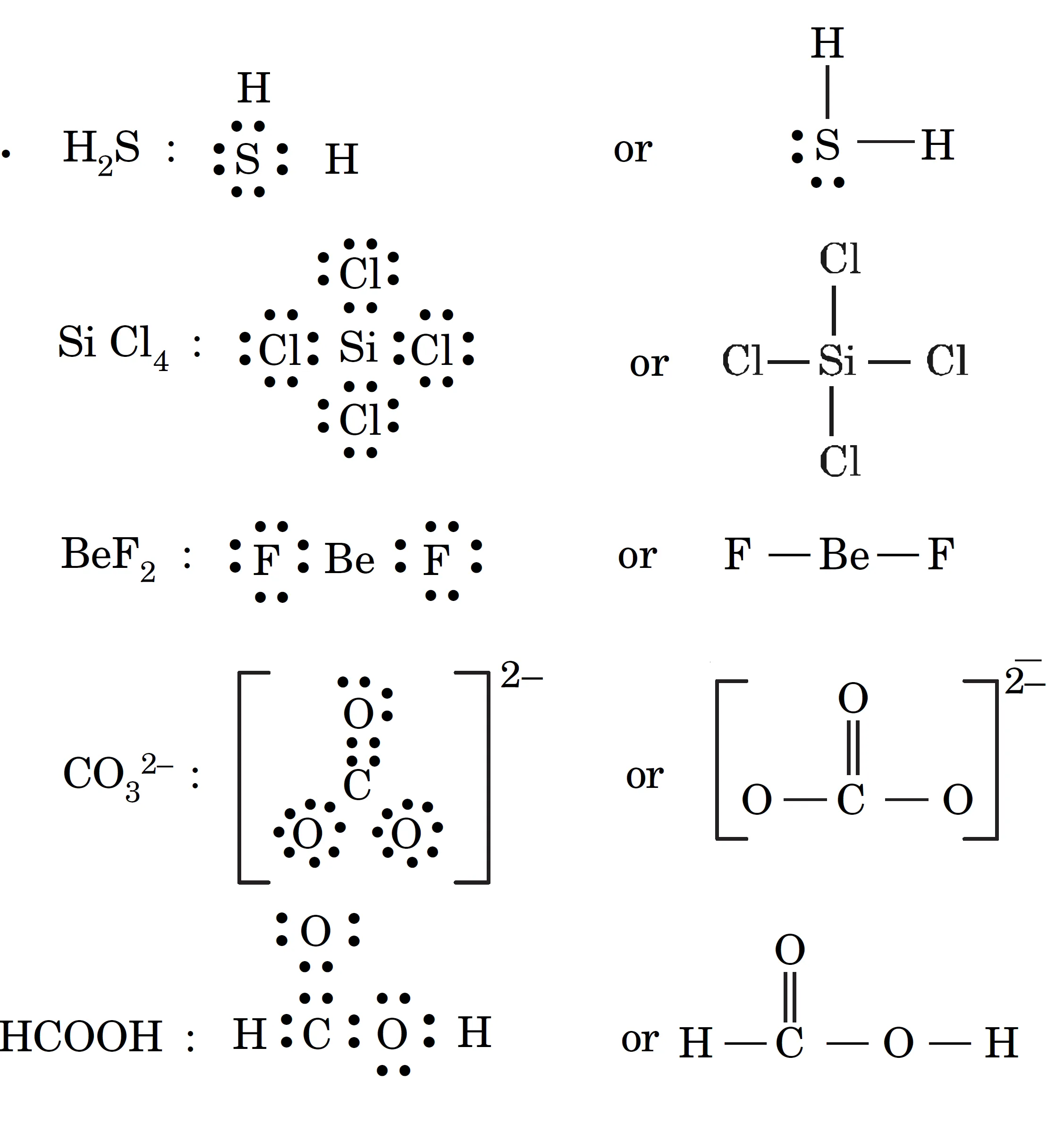
Key Takeaways:
- Lewis structures help in visualizing bonds and lone pairs.
- They also predict molecular geometry.
Define octet rule. Write its significance and limitations.
Answer:
- Octet Rule: Atoms combine either by transfer of valence electrons from one atom to another (gaining or losing) or by sharing of valence electrons in order to complete their octets in their valence shell. This is called the octet rule.
- Significance : The octet rule helps to explain as to why different atoms combine with each other to form ionic or covalent compounds and explains stability of ionic and covalent compounds.
- Limitations: The octet rule fails to explain
- Incomplete octet : The formation of molecules in which the central atom has less than eight electrons in the valence shell such as BeCl2, BF3
- Expanded octet : The formation of molecules in which the central atom has more than eight electrons in the valence shell such as PF5, SF6.
- Noble gas compounds : The formation of compounds of noble gases especially xenon and krypton such as XeF2, XeF6.
- Odd-electron molecules (e.g., NO, NO2).
Key Takeaways:
- Octet Rule is useful but not universal.
- Several molecules do not obey it.
Write the favourable factors for the formation of ionic bond.
Answer:
- Low ionization enthalpy of the metal atom.
- High electron gain enthalpy of the non-metal atom.
- High lattice enthalpy of the compound formed.
Key Takeaways:
- Ionic bonds are favoured when electron transfer is easy and lattice energy is high.
Discuss the shape of the following molecules using the VSEPR model : BeCl2, BCl3, SiCl4, AsF5, H2S, PH3
Answer:
Shape of BeCl2
Explanation:
In beryllium chloride (BeCl2), the central atom beryllium (Be) has an electronic configuration of 1s2 2s2.
- In the excited state, Be promotes one 2s electron to 2p, forming two half-filled orbitals (1s2 2s12p1).
- Each of these orbitals forms a sigma bond with chlorine.
Bond pairs: 2
Lone pairs: 0
Since there are only two bond pairs and no lone pairs, the repulsion between bond pairs is maximum at 180°, leading to a linear shape.
Shape: Linear
Bond angle: 180°
Key Takeaways:
- Bond pairs = 2, Lone pairs = 0
- Geometry = Linear due to equal repulsion between bond pairs.
Shape of BCl3
Explanation:
In boron trichloride (BCl3), the central atom boron (B) has an excited state electronic configuration of (1s2 2s12px12py1)..
- It forms three sigma bonds with three chlorine atoms using sp2 hybrid orbitals.
- There are no lone pairs on boron.
Bond pairs: 3
Lone pairs: 0
The three bond pairs repel each other equally in a plane, resulting in 120° bond angles and a trigonal planar shape.
Shape: Trigonal Planar
Bond angle: 120°
Key Takeaways:
- Bond pairs = 3, Lone pairs = 0
- Geometry = Trigonal Planar, all atoms lie in one plane.
Shape of SiCl4
Explanation:
In silicon tetrachloride (SiCl4), the central atom silicon (Si) has four valence electrons.
- It forms four sigma bonds with four chlorine atoms.
- There are no lone pairs on silicon.
Bond pairs: 4
Lone pairs: 0
To minimize repulsion between these four bond pairs, they arrange themselves tetrahedrally with bond angles of 109.5°.
Shape: Tetrahedral
Bond angle: 109.5°
Key Takeaways:
- Bond pairs = 4, Lone pairs = 0
- Geometry = Tetrahedral due to equal bond-pair repulsion.
Shape of AsF5
Explanation:
In arsenic pentafluoride (AsF5), the central atom arsenic (As) has five valence electrons.
- It forms five sigma bonds with fluorine atoms.
- There are no lone pairs on arsenic.
Bond pairs: 5
Lone pairs: 0
The five pairs of bonding electrons arrange themselves in a trigonal bipyramidal geometry to minimize repulsion:
- Three fluorine atoms in one plane (equatorial positions).
- Two fluorine atoms above and below this plane (axial positions).
Shape: Trigonal Bipyramidal
Bond angles: 120° (equatorial) and 90° (axial)
Key Takeaways:
- Bond pairs = 5, Lone pairs = 0
- Geometry = Trigonal Bipyramidal with two types of bond angles.
Shape of H2S
Explanation:
In hydrogen sulfide (H2S), the central atom sulfur (S) has six valence electrons.
- Two electrons are used to form two sigma bonds with hydrogen atoms.
- The remaining four electrons (two pairs) remain as lone pairs.
Bond pairs: 2
Lone pairs: 2
The strong repulsion between lone pairs pushes the bond pairs closer, resulting in a bent or V-shaped geometry.
Shape: Bent or V-shaped
Bond angle: ≈ 92°
Key Takeaways:
- Bond pairs = 2, Lone pairs = 2
- Lone pair–bond pair repulsion > bond pair–bond pair repulsion, giving bent shape.
Shape of PH3
Explanation:
In phosphine (PH3), the central atom phosphorus (P) has five valence electrons.
- Three electrons are used to form three sigma bonds with hydrogen atoms.
- The remaining two electrons form one lone pair.
Bond pairs: 3
Lone pairs: 1
The presence of a lone pair causes repulsion, which pushes the three P–H bonds downward, resulting in a trigonal pyramidal shape.
Shape: Trigonal Pyramidal
Bond angle: ≈ 93.5°
Key Takeaways:
- Bond pairs = 3, Lone pairs = 1
- Shape = Trigonal Pyramidal due to lone pair repulsion on top.
Although geometries of NH3 and H2O molecules are distorted tetrahedral, bond angle in water is less than that of ammonia. Discuss
Answer:
NH3 molecule has one lone pair while H2O has two lone pairs of electrons. Due to the presence of lone pairs the geometries of NH3 and H2O are distorted. Due to the presence of stronger lp–bp repulsion than bp–bp repulsion, the bond angle in NH3 is reduced from normal tetrahedral bond angle (109.5°) to 107°. In case of H2O, two lp of electrons force the O—H bonds more closely than N–H bonds in NH3. So the bond angle decreases to a larger extent i.e., to 104.5°.
- In NH₃, there is one lone pair on N atom.
- In H₂O, there are two lone pairs on O atom → stronger repulsion on bond pairs → smaller bond angle.
Key Takeaways:
- More lone pairs → greater bond pair repulsion → smaller bond angle.
How do you express the bond length in terms of bond order ?
Answer:
- Greater the bond order → stronger the bond and smaller the bond length
Example: Bond order of O₂ = 2 → double bond is stronger than single bond.
Key Takeaways:
- Bond order directly measures bond strength and stability.
Define bond length
Answer:
- Bond length is defined as the average distance between the centres of the nuclei of two bonded atoms in a molecule.
- The equilibrium distance between the nuclei of two bonded atoms is called bond length.
Key Takeaways:
- Bond length = balance point between attraction and repulsion.
- Shorter bond length = stronger bond.


