Anand Classes presents NCERT Exemplar Solutions for questions 31 to 40 (Short Answer Type Questions) from the chapter Classification of Elements and Periodicity in Properties for Class 11 Chemistry. These solutions are prepared to assist students in mastering periodic properties, element classification, and trends with in-depth explanations, diagrams, and solved examples to enhance learning and support board exam success. Click the print button to download study material and notes.
Q. 31 Illustrate by taking examples of transition elements and non-transition elements that oxidation states of elements are largely based on electronic configuration.
Ans. The oxidation state of an element depends mainly on the number of valence electrons (outermost shell electrons).
It can also be predicted as (8 − number of valence electrons) for p-block elements.
Let us see through examples:
(A) Non-transition elements (s- and p-block):
- Alkali metals (Group 1):
- General valence shell configuration: $ns^1$
- They can lose 1 electron → Oxidation state = +1
- Example: Na ($1s^22s^22p^63s^1$) → Na$^+$
- Alkaline earth metals (Group 2):
- General valence shell configuration: $ns^2$
- They can lose 2 electrons → Oxidation state = +2
- Example: Mg ($[Ne]3s^2$) → Mg$^{2+}$
- Group 13 elements:
- General configuration: $ns^2 np^1$
- Oxidation states: +3 (common), sometimes +1 (in heavier elements due to inert pair effect).
- Example: Al → +3; Tl → +1 and +3
- Group 14 elements:
- General configuration: $ns^2 np^2$
- Oxidation states: +4 (common), sometimes +2 (in heavier elements).
- Example: C → +4 (CO$_2$), Sn → +2, +4
- Group 15 elements:
- General configuration: $ns^2 np^3$
- Oxidation states: –3, +3, +5
- Example: N shows +1, +2, +4, +5 (like in N$_2$O, NO, NO$_2$, HNO$_3$).
- Group 16 elements:
- General configuration: $ns^2 np^4$
- Oxidation states: –2, +2, +4, +6
- Example: O → –2, S → +2, +4, +6
- Group 17 (halogens):
- General configuration: $ns^2 np^5$
- Common oxidation state = –1
- Heavier halogens (Cl, Br, I) also show +1, +3, +5, +7.
- Group 18 (noble gases):
- General configuration: $ns^2 np^6$
- Oxidation state = 0 (inert), but heavier ones (Xe) can show +2, +4, +6, +8.
(B) Transition elements (d-block):
- General configuration: $(n-1)d^{1-10} ns^{0-2}$
- They exhibit variable oxidation states because both (n-1)d and ns electrons can participate in bonding.
- Example:
- Fe → +2 (loss of 2 electrons from 4s), +3 (loss of 2 from 4s + 1 from 3d).
- Mn → +2, +3, +4, +6, +7.
Thus, non-transition elements usually show fixed oxidation states based on their group number, while transition elements show variable oxidation states due to the availability of both d- and s-electrons.
Q. 32 Nitrogen has positive electron gain enthalpy whereas oxygen has negative. However, oxygen has lower ionisation enthalpy than nitrogen. Explain.
Ans.
Step 1: Electron gain enthalpy (ΔegH):
- Nitrogen (Z = 7):
Electronic configuration = $1s^2 2s^2 2p^3$ - The 2p orbital is exactly half-filled, which is very stable.
- Adding an electron would disturb this stability, so energy must be supplied → positive electron gain enthalpy.
- Oxygen (Z = 8):
Electronic configuration = $1s^2 2s^2 2p^4$ - The p-orbitals are not half-filled.
- Addition of an electron completes pairing in one orbital → releases energy → negative electron gain enthalpy.
Step 2: Ionisation enthalpy (IE):
- Nitrogen:
Removing an electron from half-filled 2p orbitals is difficult due to stability → higher IE. - Oxygen:
Removing one electron from 2p$^4$ gives oxygen a stable 2p$^3$ (half-filled) configuration → lower IE.
Hence,
- N: Positive ΔegH, Higher IE
- O: Negative ΔegH, Lower IE
Q. 33 First member of each group of representative elements (s- and p-block) shows anomalous behaviour. Illustrate with two examples.
Ans.
The first member of each group shows anomalous behaviour due to:
- Small size
- High ionisation enthalpy
- High electronegativity
- Absence of d-orbitals
Examples:
(A) s-block (alkali metals):
- Lithium differs from other alkali metals.
- Li compounds have more covalent character (due to small size & high polarising power).
- Li forms Li$_3$N (lithium nitride) while other alkali metals do not form stable nitrides.
(B) p-block:
- Carbon (first member of Group 14) shows anomaly.
- C forms strong covalent bonds due to small size and ability to form $pπ−pπ$ multiple bonds.
- Si, Ge, Sn, Pb cannot form strong $pπ−pπ$ bonds due to larger size.
- Boron (first member of Group 13) also shows different chemistry than Al, Ga, In.
Q. 34 p-block elements form acidic, basic and amphoteric oxides. Explain with examples and reactions with water.
Ans.
(A) General trend across a period:
- From left to right → oxides become more acidic.
- Example:
- 2nd period: B$_2$O$_3$ < CO$_2$ < N$_2$O$_3$ (acidic strength increases)
- 3rd period: Al$_2$O$_3$ < SiO$_2$ < P$_2$O$_5$ < SO$_3$ < Cl$_2$O$_7$
(B) Down a group:
- Acidic character decreases, basic character increases.
Examples of oxides:
- Acidic oxides:
- Non-metal oxides like CO$_2$, SO$_2$, P$_2$O$_5$, Cl$_2$O$_7$.
- Reactions:
$$SO_3 + H_2O → H_2SO_4$$
$$P_2O_5 + 6H_2O → 4H_3PO_4$$
- Basic oxides:
- Metallic oxides like Na$_2$O, CaO, Tl$_2$O.
- Reactions:
$$Na_2O + H_2O → 2NaOH$$
$$CaO + H_2O → Ca(OH)_2$$
- Amphoteric oxides:
- Example: Al$_2$O$_3$, ZnO.
- Reacts with acids:
$$Al_2O_3 + 6HCl → 2AlCl_3 + 3H_2O$$ - Reacts with bases:
$$Al_2O_3 + 2NaOH → 2NaAlO_2 + H_2O$$
Key point: Higher oxidation state → more acidic oxide.
E.g., SO$_3$ (S in +6) is more acidic than SO$_2$ (S in +4).
Q. 35 Why is the first ionisation enthalpy of Na lower than Mg, but the second ionisation enthalpy of Na higher than Mg?
Ans.
Step 1: First ionisation enthalpy (IE$_1$):
- Na (Z = 11): $1s^22s^22p^63s^1$
- Outer electron in 3s, low nuclear charge → easy to remove.
- Mg (Z = 12): $1s^22s^22p^63s^2$
- Has 2 outer electrons, stronger nuclear charge → harder to remove.
Thus, IE$_1$(Na) < IE$_1$(Mg).
Step 2: Second ionisation enthalpy (IE$_2$):
- Na$^+$ = [Ne] → stable noble gas configuration. Removing another electron breaks stability → very high IE$_2$.
- Mg$^+$ = [Ne]3s$^1$ → still has 1 valence electron in 3s. Removing it is easier than removing from noble gas core.
Thus, IE$_2$(Na) > IE$_2$(Mg).
Q. 36 Define exothermic and endothermic reactions with examples.
Ans.
Exothermic reaction: Reaction accompanied by evolution of heat.
- Heat released = negative ΔH.
Examples:
- $$C(s) + O_2(g) → CO_2(g) + 393.5\ \text{kJ}$$
- $$H_2(g) + \tfrac{1}{2}O_2(g) → H_2O(l); \ ΔH = -285.8\ \text{kJ mol}^{-1}$$
Endothermic reaction: Reaction accompanied by absorption of heat.
- Heat absorbed = positive ΔH.
Examples:
- $$C(s) + H_2O(g) → CO(g) + H_2(g); \ ΔH = +131.4\ \text{kJ}$$
- $$N_2(g) + 3H_2(g) → 2NH_3(g); \ ΔH = +92.4\ \text{kJ mol}^{-1}$$
Q. 37 Arrange N, P, O and S in the order of:
(i) increasing first ionisation enthalpy.
(ii) increasing non-metallic character.
Ans.
Elements:
- 2nd period: N (Group 15), O (Group 16)
- 3rd period: P (Group 15), S (Group 16)
(i) Ionisation enthalpy order:
- Down a group → decreases (N > P, O > S).
- Across a period → increases (P < S, N < O).
- But N has higher IE than O due to half-filled p-orbitals.
Final order:
$$S < P < O < N$$
(ii) Non-metallic character:
- Across a period → increases (P < S, N < O).
- Down a group → decreases (N > P, O > S).
Final order:
$$P < S < N < O$$
Q. 38 Explain the deviation in ionisation enthalpy of some elements from the general trend by using given figure.
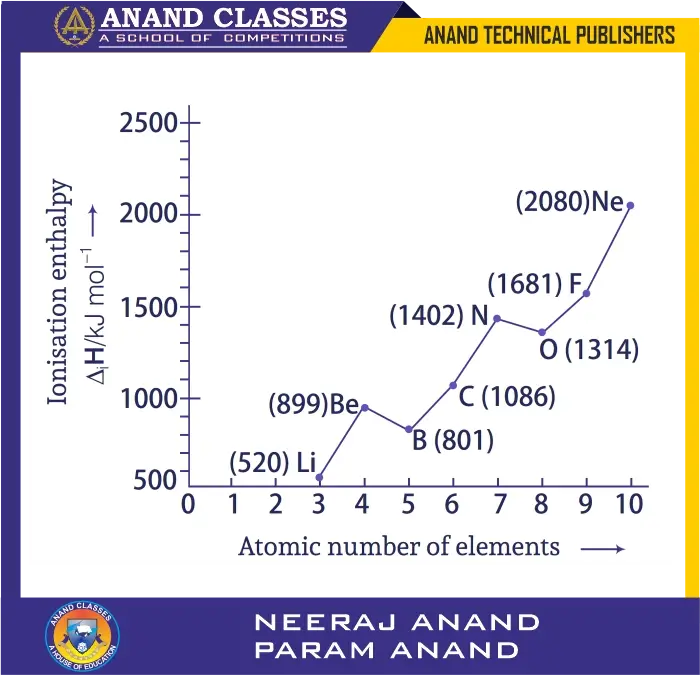
Ans.
- General trend: IE increases across a period.
- Deviations:
- Boron < Beryllium:
- Be has stable filled 2s$^2$, requires more energy.
- B has 2p$^1$, easier to remove.
- Nitrogen > Oxygen:
- N has half-filled stable p$^3$, so IE is higher.
- O has p$^4$, electron pairing causes repulsion, easier to remove.
Q. 39 (a) Electronegativity increases left to right. Why?
Ans: Across a period, nuclear charge increases and atomic radius decreases. The ability to attract electrons increases.
Across the period, the nuclear charge increases and the atomic radius decreases. As a result, the tendency of the atom of an element to attract the shared pair of electrons towards itself increases and hence the electronegativity of the element increases.
Example: In 2nd period → Li (1.0), Be (1.5), B (2.0), C (2.5), N (3.0), O (3.5), F (4.0).
(b) Ionisation enthalpy decreases top to bottom. Why?
Ans:
The ionisation enthalpy decreases regularly as we move from top to bottom, as explained below
(i) On moving down a group from top to bottom, the atomic size increases gradually due to the addition of a new principal energy shell at each succeding element. As a result, the distance between the nucleus and the valence shell increases.
In other words, the force of attraction of the nucleus for the valence electrons
decreases and hence the ionisation enthalpy should decrease.
(ii) With the addition of new shells, the number of inner shell which shield the valence electrons from the nucleus increases. In other words, the shielding effect or the screening effect increases.
As a result, the force of attraction of the nucleus for the valence electrons further decreases and hence the ionisation enthalpy should decrease.
(iii) Further, in a group from top to bottom nuclear charge increases with increase in atomic number. As a result, the force of attraction of the nucleus for the valence electrons increases and hence the ionisation enthalpy should increase.
The combined effect of the increase in atomic size and screening effect more than compensate the effect of the increased nuclear charge. Consequently, the valence electrons become less and less firmly held by the nucleus and hence the ionisation enthalpy gradually decreases as we move down the group.
- Atomic size increases → nucleus farther from valence electrons.
- Shielding effect increases → outer electrons feel less attraction.
- Effect of nuclear charge increase is less significant.
Hence, IE decreases down a group.
Q. 40 How does metallic and non-metallic character vary across a period?
Ans.
As we move from left to right in a period, the number of valence electrons increases by one at each succeeding element but the number of shells remains same. Due to this effective nuclear charge increases. More is the effective nuclear charge, more is the attraction between nuclei and electron.
Hence, the tendency of the element to lose electrons decreases, this results in decrease in metallic character. Furthermore, the tendency of an element to gain electrons increases with increase in effective nuclear charge, so non-metallic character increases on moving from left to right in a period
- Across a period:
- Effective nuclear charge increases (more protons, same shells).
- Electrons are more tightly held.
- Tendency to lose electrons decreases → metallic character decreases.
- Tendency to gain electrons increases → non-metallic character increases.
Example (Period 2): Li (metallic) → Be → B → C → N → O → F (non-metallic).
📚 Buy Study Material & Join Our Coaching
For premium study materials specially designed for JEE, NEET, NDA, and CBSE/ICSE Classes, visit our official study material portal:
👉 https://publishers.anandclasses.co.in/
For NDA Study Material, Click Here
For SSC Study Material, Click Here
To enroll in our offline or online coaching programs, visit our coaching center website:
👉 https://anandclasses.co.in/
📞 Call us directly at: +91-94631-38669
💬 WhatsApp Us Instantly
Need quick assistance or want to inquire about classes and materials?
📲 Click below to chat instantly on WhatsApp:
👉 Chat on WhatsApp
🎥 Watch Video Lectures
Get access to high-quality video lessons, concept explainers, and revision tips by subscribing to our official YouTube channel:
👉 Neeraj Anand Classes – YouTube Channel


