Anand Classes explains the Effects of Hydrogen Bonding on Boiling Point, Solubility, Volatility, Association, and Physical State of different compounds. Students will learn how intermolecular hydrogen bonding increases molecular attraction, leading to higher boiling points, greater solubility in polar solvents, and lower volatility, while intramolecular hydrogen bonding often reduces solubility and affects the physical state of substances. The topic also discusses how hydrogen bonding causes association in liquids like water and hydrogen fluoride. This concept is important for Class 11, Class 12, JEE, and NEET Chemistry, and is supported with diagrams, examples, MCQs, Q&A, Assertion Reason, and Case Study questions. Click the print button to download study material and notes.
How Does Hydrogen Bonding Influence on the Physical Properties of Compounds ?
Hydrogen bonding has important effect on many physical properties such as melting point, boiling point, volatility and solubility of the compounds. The main characteristics of compounds having hydrogen bonds are given below.
How Does Hydrogen Bonding Influence Association of Molecules?
Hydrogen bonding causes intermolecular association of molecules.
- Due to hydrogen bonding, two or more molecules exist as associated molecules.
- For example, carboxylic acids (RCOOH) form dimers even in the vapour state:
(RCOOH)2
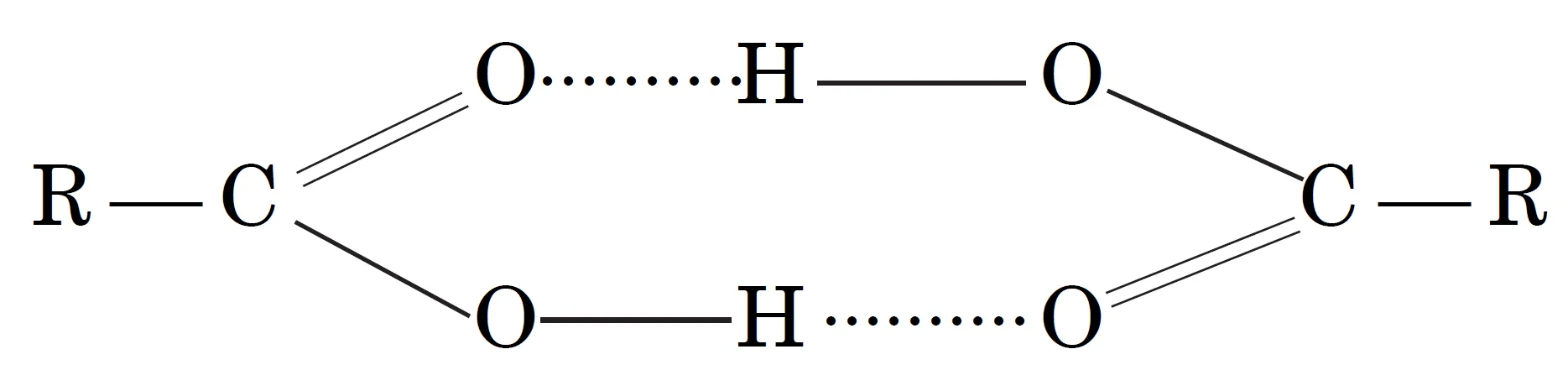
- This leads to an increase in effective size and molecular mass of the compound.
Key Takeaways:
- Hydrogen bonding leads to molecular association.
- Carboxylic acids exist as dimers due to hydrogen bonding.
Why Do Compounds with Hydrogen Bonding Have Higher Melting and Boiling Points?
Compounds containing hydrogen bonds have abnormally high melting and boiling points.
- Reason: Hydrogen bonds create strong electrostatic force of attractions between molecules.
- More energy is required to break these bonds, leading to higher melting and boiling points.
Example – Hydrides of Groups 14, 15, 16, 17:
| Hydride | m.p. (K) | b.p. (K) |
|---|---|---|
| Group 14 | ||
| CH4 | 89.0 | 111.5 |
| SiH4 | 88.0 | 161.2 |
| GeH4 | 108.0 | 183.0 |
| SnH4 | 123.0 | 221.0 |
| Group 15 | ||
| NH3 | 195.5 | 239.6 |
| PH3 | 138.0 | 185.0 |
| AsH3 | 159.0 | 218.0 |
| SbH3 | 184.0 | 256.0 |
| Group 16 | ||
| H2O | 273.0 | 373.0 |
| H2S | 190.3 | 211.2 |
| H2Se | 209.0 | 231.0 |
| H2Te | 222.0 | 271.0 |
| Group 17 | ||
| HF | 180.7 | 292.4 |
| HCl | 161.0 | 189.4 |
| HBr | 184.5 | 206.0 |
| HI | 222.2 | 237.0 |
- The influence of hydrogen bonding on melting and boiling points can be understood comparing the melting and boiling points of the hydrides of elements of groups 14, 15, 16 and 17 (See Table).
- It is clear from Table, that the melting and boiling points increase as the molecular mass increases in group 14. This is mainly due to the fact that as the size of elements of group 14 increases, the number of electrons also increases. As a result, van der Waals’ forces also increase and therefore, melting and boiling points increase. However, hydrides of groups 15, 16 and 17 do not show this trend. In these groups, the melting and boiling points also increase with increasing molecular mass with the exception of first member. But, the first members NH3 (group 15), H2O (group 16) and HF (group 17) have abnormally high melting and boiling points.
- Group 14 hydrides: m.p. and b.p. increase with molecular mass due to van der Waals’ forces.
- Groups 15, 16, 17 hydrides: first members (NH3, H2O, HF) show abnormally high boiling points due to hydrogen bonding.
- HF vs H2O: Although HF has stronger H-bonds, H2O boils at higher temperature than HF because each each H2O molecule is bonded to four other H2O molecules through hydrogen bonds whereas each HF molecule is bonded to two other HF molecules through hydrogen bonds.
Key Takeaways:
- Hydrogen bonding increases melting and boiling points.
- NH3, H2O, and HF show anomalously high values compared to heavier hydrides.
How Does Hydrogen Bonding Affect Physical State of Substances?
Hydrogen bonding strongly influences whether a substance exists as solid, liquid, or gas.
For example, both O and S belong to same group but H2O is a liquid at ordinary temperature, while H2S is a gas. This is explained on the basis of electronegativity values.
Electronegativity values:
- O = 3.5, H = 2.1 → strong polarity → strong H-bonding.
- S = 2.5, H = 2.1 → weak polarity → negligible H-bonding.
In water, oxygen is highly electronegative so that it forms hydrogen bonds. As a result, the molecules of H2O get associated with one another and this raises the boiling point of water. Consequently, water exists as liquid at room temperature.
On the other hand, the electronegativity difference of atoms in H2S is less and hydrogen bonding in H2S is almost negligible. As a result, H2S is not associated and exists as a gas at room temperature.
Key Takeaways:
- Example: Oxygen and sulfur belong to the same group.
- H2O → liquid at room temperature due to strong H-bonding.
- H2S → gas at room temperature (negligible H-bonding).
- Stronger hydrogen bonding → higher boiling point → liquid state.
- Weaker hydrogen bonding → gaseous state.
How Does Hydrogen Bonding Influence Solubility?
Hydrogen bonding enhances solubility of compounds in water.
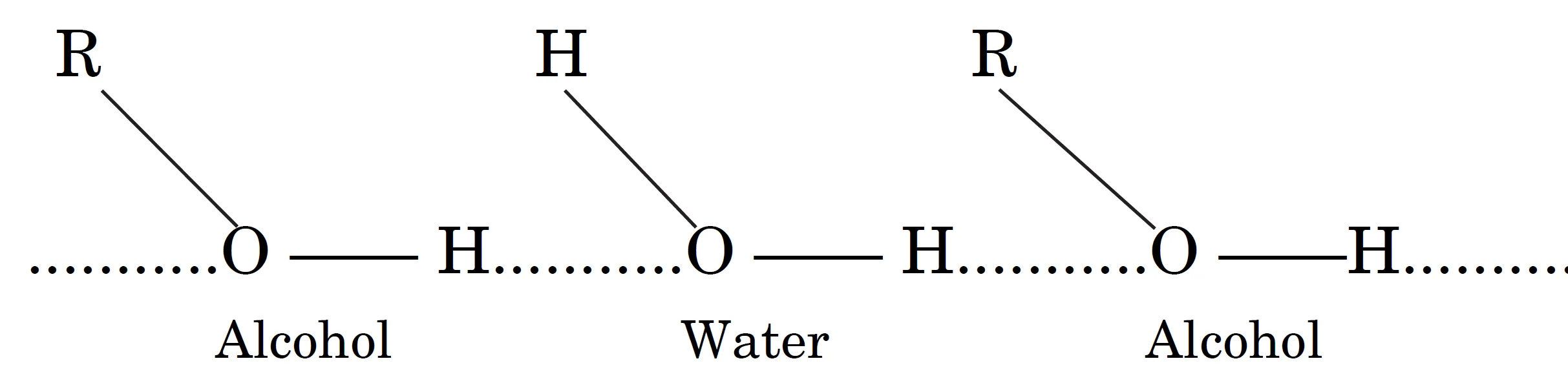
- Example: Alcohols dissolve readily in water by forming hydrogen bonds with H2O molecules.
Key Takeaways:
- Compounds capable of H-bonding with water show high solubility.
- Explains solubility of alcohols, amines, sugars.
How Does Hydrogen Bonding Influence Volatility?
Compounds with hydrogen bonding have lower volatility.
- Because their boiling points are high, they do not vaporize easily.
- Examples: NH3, H2O, and HF are less volatile than corresponding group hydrides.
Trends in enthalpy of vaporisation: The enthalpies of vaporisation follows almost the same pattern as shown by their melting points and boiling points. For example, in group 17, the enthalpies of vaporisation decrease in the order
- Group 17: HCl < HBr < HI < HF
- Group 16: H2S < H2Se < H2Te < H2O
- Group 15: PH3 < AsH3 < SbH3 < NH3
Key Takeaways:
- Hydrogen bonding lowers volatility by raising boiling point.
- Explains high enthalpy of vaporisation for H2O, NH3, HF.
Why Does HF Have a Higher Boiling Point Than HCl and HBr?
Order: HF > HBr > HCl
- Reason: HF has extensive hydrogen bonding compared to HCl and HBr, which lack strong H-bonding.
Key Takeaways:
- HF’s strong hydrogen bonding gives it highest boiling point among halogen hydrides.
Do o-Nitrophenol and p-Nitrophenol Have Hydrogen Bonding ? Explain which of the two has higher boiling point?
Yes, both have hydrogen bonding, but of different types:
- Intramolecular hydrogen bonding : o-Nitrophenol has Intramolecular hydrogen bonding (within same molecule). No association of molecules possible. But due to larger distance between —NO2 and —OH groups in p-nitrophenol, there is no interamolecular hydrogen bonding.
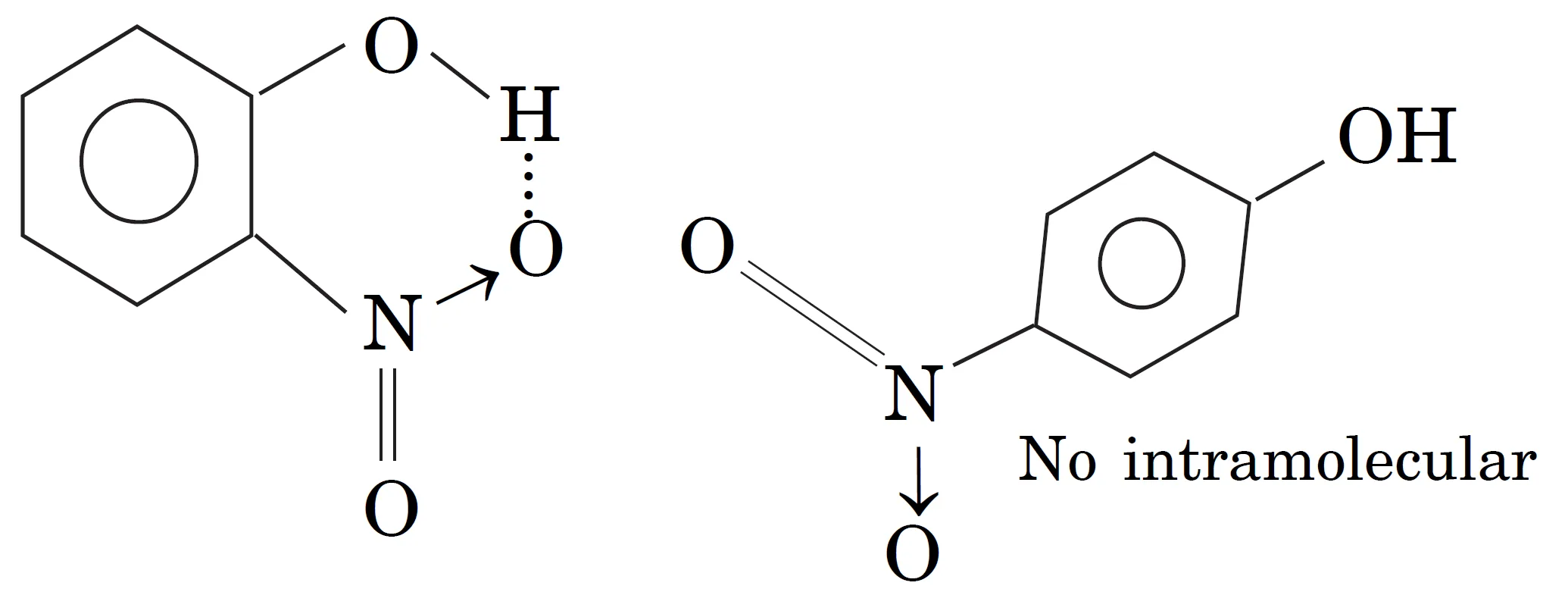
- p-Nitrophenol has Intermolecular hydrogen bonding between molecules). Therefore it exists as associated molecules. In o-nitrophenol, no intermolecular hydrogen bonding is possible
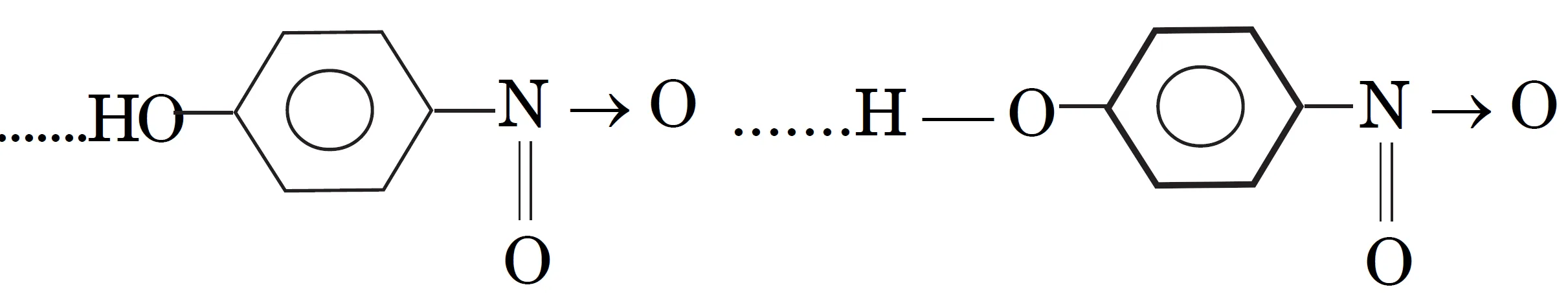
Due to associated nature of p-nitrophenol, it is less volatile and has high boiling point.
Effect on boiling point:
- p-Nitrophenol has higher boiling point than o-nitrophenol.
- Reason: intermolecular H-bonding in p-nitrophenol causes molecular association, making it less volatile.
Key Takeaways:
- o-nitrophenol → intramolecular H-bonding → lower boiling point.
- p-nitrophenol → intermolecular H-bonding → higher boiling point.
Summary
Hydrogen bonding has a profound influence on the physical properties of compounds:
- Molecular Association: Dimers and clusters form due to intermolecular H-bonds (e.g., carboxylic acids).
- High Melting/Boiling Points: NH₃, H₂O, and HF show abnormally high values compared to heavier group hydrides.
- Physical State: H₂O is a liquid, but H₂S is a gas due to difference in hydrogen bonding strength.
- Solubility: Compounds like alcohols dissolve in water due to H-bond formation with H₂O.
- Volatility: H-bonding lowers volatility by increasing boiling points (H₂O, NH₃, HF).
- Special Cases: p-nitrophenol (intermolecular H-bonding) has higher boiling point than o-nitrophenol (intramolecular H-bonding).
👉 Overall, hydrogen bonding is the key factor behind anomalous boiling points, solubility trends, volatility, and molecular association. Its role explains many biological, chemical, and physical properties of matter.
Short Answer Type (SAT) Questions on Hydrogen Bonding
Q1. Why do carboxylic acids (RCOOH) exist as dimers in vapour state?
Answer: Due to intermolecular hydrogen bonding, two molecules of carboxylic acid form a cyclic dimer, increasing effective molecular mass.
Q2. Why does water (H2O) have a much higher boiling point than H2S?
Answer: Oxygen in H2O is highly electronegative, leading to strong intermolecular hydrogen bonding. In contrast, H2S shows negligible hydrogen bonding due to lower electronegativity of sulfur.
Q3. Why is p-nitrophenol less volatile than o-nitrophenol?
Answer: p-nitrophenol shows intermolecular hydrogen bonding, leading to association and higher boiling point. o-nitrophenol shows intramolecular hydrogen bonding, so no association is possible.
Q4. Why does HF have a higher boiling point than HCl?
Answer: HF molecules are associated through strong intermolecular hydrogen bonding, while HCl does not show hydrogen bonding.
Multiple Choice Questions (MCQs) on Hydrogen Bonding
Q5. Which of the following hydrides shows abnormally high boiling point due to hydrogen bonding?
(a) PH3
(b) H2O
(c) H2S
(d) HCl
Answer: (b) H₂O
Explanation: Water forms a tetrahedral hydrogen-bonded network, raising its boiling point.
Q6. Which type of hydrogen bonding is responsible for higher solubility of alcohols in water?
(a) Intramolecular
(b) Intermolecular
(c) Ionic bonding
(d) van der Waals forces
Answer: (b) Intermolecular
Explanation: Alcohols form intermolecular hydrogen bonds with water, making them highly soluble.
Q7. Arrange the following in decreasing order of boiling points: HF, HCl, HBr
(a) HF > HCl > HBr
(b) HCl > HBr > HF
(c) HF > HBr > HCl
(d) HBr > HF > HCl
Answer: (c) HF > HBr > HCl
Explanation: HF has strong hydrogen bonding, while HBr and HCl lack significant hydrogen bonding.
Q8. Which nitrophenol shows intramolecular hydrogen bonding?
(a) o-nitrophenol
(b) p-nitrophenol
(c) Both
(d) None
Answer: (a) o-nitrophenol
Explanation: In o-nitrophenol, –OH and –NO₂ are close, forming intramolecular H-bonds, while p-nitrophenol shows intermolecular H-bonding.
Assertion–Reason Type Questions (ARQs) on Hydrogen Bonding
Q9.
Assertion (A): H2O exists as a liquid at room temperature, while H₂S exists as a gas.
Reason (R): H2O molecules are linked by intermolecular hydrogen bonding, while H2S lacks hydrogen bonding.
Options:
(a) Both A and R are true, and R is the correct explanation of A.
(b) Both A and R are true, but R is not the correct explanation of A.
(c) A is true, R is false.
(d) A is false, R is true.
Answer: (a) Both A and R are true, and R explains A.
Q10.
Assertion (A): p-nitrophenol has a higher boiling point than o-nitrophenol.
Reason (R): o-nitrophenol shows intramolecular hydrogen bonding, while p-nitrophenol shows intermolecular hydrogen bonding.
Options:
(a) Both A and R are true, and R is the correct explanation of A.
(b) Both A and R are true, but R is not the correct explanation of A.
(c) A is true, R is false.
(d) A is false, R is true.
Answer: (a) Both A and R are true, and R explains A.
Q11.
Assertion (A): HF has a much higher boiling point than HCl.
Reason (R): HF molecules are associated through strong intermolecular hydrogen bonding.
Options:
(a) Both A and R are true, and R is the correct explanation of A.
(b) Both A and R are true, but R is not the correct explanation of A.
(c) A is true, R is false.
(d) A is false, R is true.
Answer: (a) Both A and R are true, and R explains A.
Case Study on Hydrogen Bonding
Read the passage and answer the questions:
Hydrogen bonding plays a vital role in influencing boiling point, melting point, solubility, volatility, and physical state. Compounds like HF, H2O, and NH3 show abnormally high boiling points compared to their heavier congeners because of intermolecular hydrogen bonding. Alcohols dissolve in water due to hydrogen bonding, while p-nitrophenol has a higher boiling point than o-nitrophenol because of intermolecular association.
Q12.1. Why is the boiling point of H2O higher than that of HF, even though HF has stronger hydrogen bonds?
Answer: Each H2O molecule forms four hydrogen bonds, creating a strong 3D network. In HF, each molecule forms only two hydrogen bonds.
Q12.2. Why are alcohols highly soluble in water?
Answer: They form intermolecular hydrogen bonds with water molecules.
Q12.3. Which type of hydrogen bonding is responsible for the high boiling point of p-nitrophenol?
Answer: Intermolecular hydrogen bonding.
Q12.4. Why is H2O a liquid while H2S is a gas at room temperature?
Answer: H2O has strong intermolecular hydrogen bonding, while H2S lacks significant hydrogen bonding.


