Anand Classes provides comprehensive notes on Covalent Bond and Lewis Structures along with the Octet Rule, Examples, MCQs, Assertion-Reason questions for Class 11 Chemistry. A covalent bond is formed by the sharing of electron pairs between atoms, and Lewis structures help represent these shared pairs with dots and lines. The Octet Rule explains how atoms achieve stability by attaining eight electrons in their valence shell. These notes include clear explanations, solved examples, NCERT exam-based multiple-choice questions, and assertion-reason type practice for JEE and NEET preparation. Click the print button to download the complete study material and notes in PDF format.
Conditions for Writing Lewis Dot Structures
The Lewis dot (electron dot) structures of covalent molecules are written by following these main conditions:
- Bond Formation
- Each bond is formed as a result of sharing of an electron pair between the atoms.
- Electron Contribution
- Each combining atom contributes one electron to the shared pair.
- Noble Gas Configuration
- Both combining atoms attain the stable outer shell configuration of the nearest noble gas after sharing.
- This is generally an octet (8 electrons) for all atoms except hydrogen, which requires only 2 electrons (duplet configuration like helium).
Octet Rule
The electron dot structures are written in accordance with the octet rule. According to this rule:
- Hydrogen (H) → needs 2 electrons.
- Group 17 elements (e.g., Cl) → have 7 valence electrons, share 1 electron to complete octet.
- Group 16 elements (e.g., O, S) → have 6 valence electrons, share 2 electrons.
- Group 15 elements (e.g., N) → have 5 valence electrons, share 3 electrons.
- Group 14 elements (e.g., C) → have 4 valence electrons, share 4 electrons.
Examples of Lewis Structures
1. Water (H2O)
- Oxygen (O) has 6 valence electrons.
- Shares one electron each with two hydrogen atoms.
- Each hydrogen attains 2 electrons, and oxygen attains 8 electrons (octet).
Representation:
$$
\mathrm{H:O:H}
$$
(with two lone pairs on O)
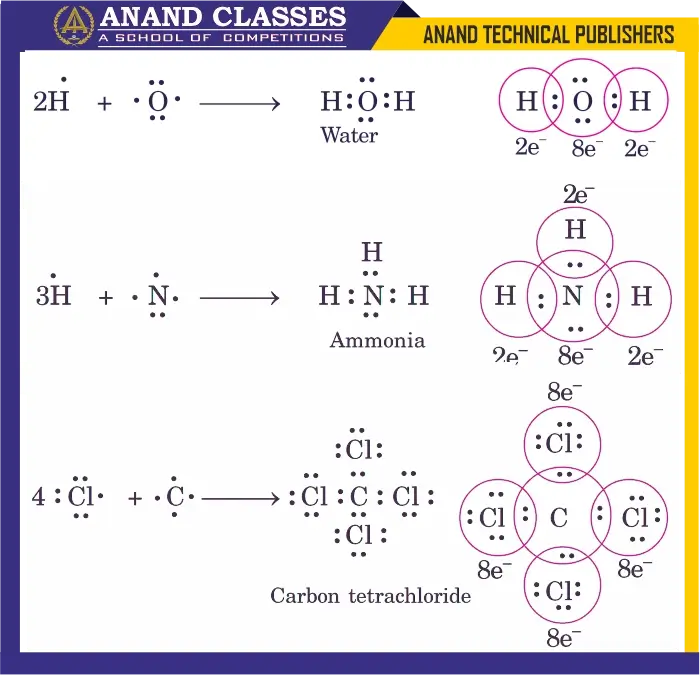
2. Ammonia (NH3)
- Nitrogen (N) has 5 valence electrons.
- Shares one electron each with three hydrogen atoms.
- Nitrogen achieves 8 electrons (octet), hydrogen achieves 2 electrons.
Representation:
$$
\mathrm{H:N:H}
$$
(with one lone pair on N)
3. Carbon Tetrachloride (CCl4)
- Carbon (C) has 4 valence electrons.
- Shares one electron each with four chlorine atoms.
- Carbon attains 8 electrons, each Cl attains 8 electrons.
Representation in the above diagram.
Key Points
- Lewis structures show all valence electrons, including lone pairs.
- Hydrogen follows the duplet rule, while other main-group elements follow the octet rule.
- Multiple bonds (double or triple) are used if sharing more electron pairs is required to satisfy the octet.
Short Answer Conceptual Types Questions (SAT) on Lewis Structures and Covalent Bond
Q1. What is a Lewis dot structure?
A. It is a diagram showing the valence electrons of atoms in a molecule, with dots representing electrons and lines or pairs representing covalent bonds.
Q2. Why does hydrogen follow the duplet rule instead of the octet rule?
A. Hydrogen has only the 1s orbital, which can accommodate 2 electrons, so it achieves stability like helium.
Q3. Which group of elements typically form single covalent bonds by sharing one electron?
A. Group 17 (Halogens), because they have 7 valence electrons and need only one electron to complete their octet.
Q4. What is the main condition for covalent bond formation?
A. Mutual sharing of electron pairs between two atoms so that each attains a noble gas configuration.
Q5. Can multiple bonds be represented in Lewis structures?
A. Yes. Double and triple covalent bonds are shown by sharing two or three electron pairs, e.g., O$_2$ (double bond) and N$_2$ (triple bond).
Multiple Choice Questions (MCQs) With Answers and Explanation on Lewis Structures and Covalent Bond
1. Which of the following molecules does not obey the octet rule?
A. NH$_3$
B. H$_2$O
C. BF$_3$
D. CCl$_4$
Answer: C. BF3 (boron has only 6 electrons around it)
2. The number of covalent bonds in an NH$_3$ molecule is:
A. 1
B. 2
C. 3
D. 4
Answer: C. 3
3. In a Lewis structure, the shared pair of electrons is shown by:
A. Dots only
B. A single line only
C. Either dots or a single line
D. Crosses
Answer: C. Either dots or a single line
4. The central atom in CCl$_4$ shares how many electrons?
A. 2
B. 4
C. 6
D. 8
Answer: B. 4 (one with each chlorine)
5. Which of the following has a lone pair on the central atom?
A. CH$_4$
B. NH$_3$
C. CCl$_4$
D. SiCl$_4$
Answer: B. NH$_3$
Assertion Reason Type Questions With Answers and Explanation on Lewis Structures and Covalent Bond
Assertion (A): Hydrogen molecule (H$_2$) is stable because each hydrogen atom attains a duplet configuration.
Reason (R): Each hydrogen atom shares one electron to complete the first shell.
- (A) Both A and R are true, and R is the correct explanation of A.
- (B) Both A and R are true, but R is not the correct explanation of A.
- (C) A is true, R is false.
- (D) A is false, R is true.
Answer: A
Assertion (A): Oxygen atom in water molecule shares two electron pairs.
Reason (R): Oxygen needs two electrons to complete its octet.
Answer: A
Case Study based on Lewis Structures and Covalent Bond
Read the passage and answer the questions below:
Carbon forms a large number of covalent compounds because it has four valence electrons and needs four more to complete its octet. It shares electrons with other atoms to form stable molecules such as methane (CH$_4$). In methane, carbon forms four single covalent bonds with hydrogen atoms. Hydrogen requires only two electrons (duplet) for stability.
Questions:
- Why does carbon not form C$^{4+}$ or C$^{4-}$ ions?
Answer: Because gaining or losing four electrons requires a very large amount of energy; sharing is energetically more favorable. - How many shared pairs of electrons are present in a methane molecule?
Answer: Four shared pairs (one for each C–H bond). - What is the electronic configuration of carbon before bond formation?
Answer: 1s$^2$ 2s$^2$ 2p$^2$ - Which rule explains the stability of hydrogen atoms in methane?
Answer: Duplet rule.


