Anand Classes explains the Bond Order and Stability of N₂, N₂⁺, O₂, Oxide (O₂²⁻), Peroxide (O₂²⁻), and Superoxide (O₂⁻) ions, along with the reason why Ne₂ molecule does not exist, based on the Molecular Orbital Theory (MOT). Students will learn how the distribution of bonding and antibonding electrons in molecular orbitals affects the bond order, stability, and magnetic character of these diatomic molecules and ions. Detailed energy level diagrams, electronic configurations, and bond order calculations are included to strengthen conceptual clarity. This topic is crucial for Class 11, Class 12, JEE, and NEET Chemistry preparation and comes with solved examples, MCQs, Q&A, Assertion Reason, and Case Study questions. Click the print button to download study material and notes.
What is the Molecular Orbital Theory of Nitrogen Molecule (N2)?
The electronic configuration of nitrogen atom is:
$$1s^2 \, 2s^2 \, 2p_x^1 \, 2p_y^1 \, 2p_z^1$$
Nitrogen molecule (N2) has 14 electrons.
The molecular orbital (MO) electronic configuration of the molecule is:
$$N_2 : KK \, (\sigma 2s)^2 \, (\sigma^* 2s)^2 \, (\pi 2p_x)^2 \, (\pi 2p_y)^2 \, (\sigma 2p_z)^2$$
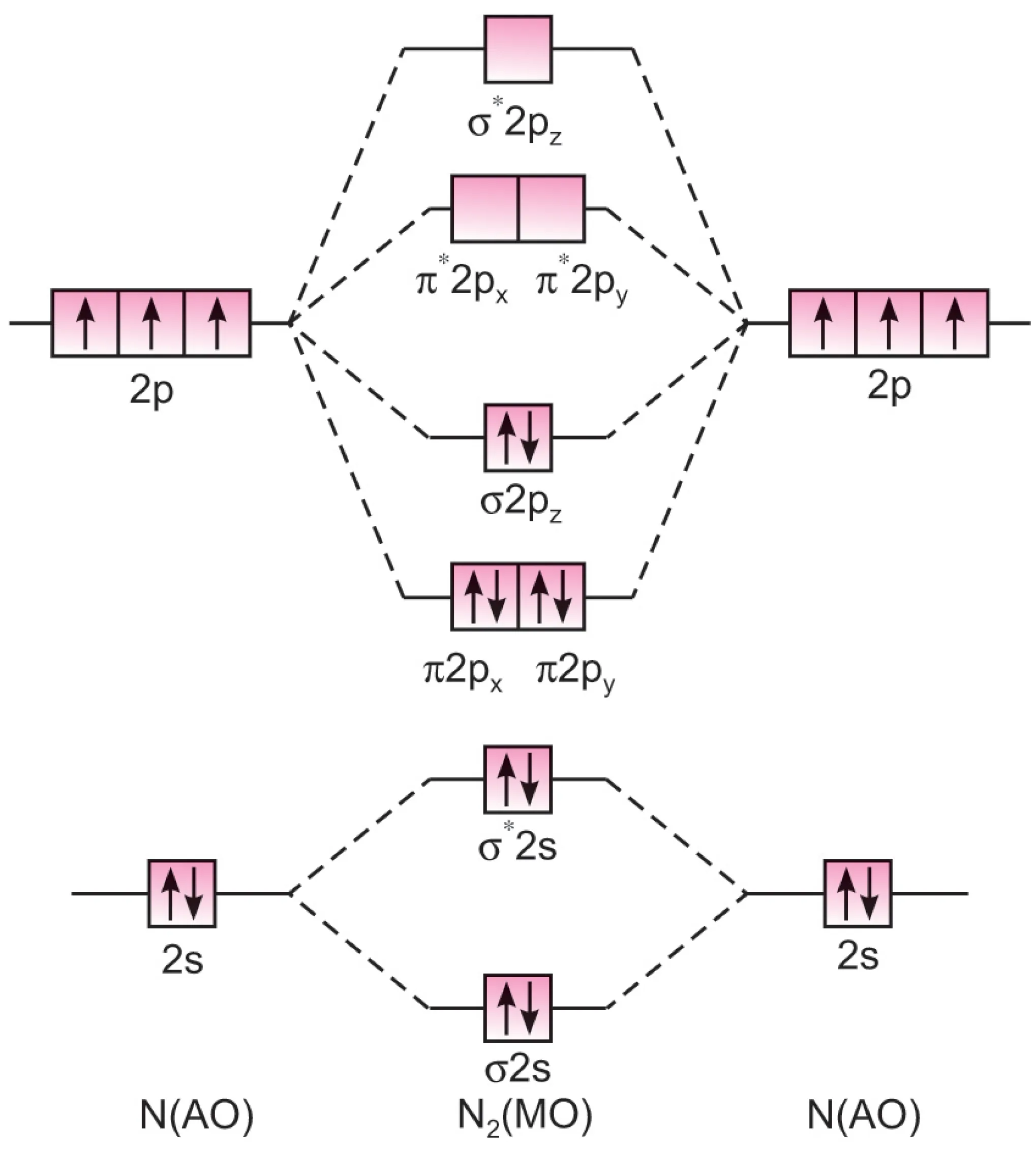
N2 molecule
Bond order is given by:
$$\text{Bond order} = \frac{(N_b – N_a)}{2} = \frac{8-2}{2} = 3$$
Thus, nitrogen molecule has three bonds – one $\sigma$ and two $\pi$ bonds.
- Bond dissociation energy: 945 kJ mol⁻¹
- Bond length: 110 pm
- Magnetic nature: Diamagnetic (no unpaired electrons).
Key Takeaways:
- Bond order of N2 = 3
- Triple bond present (1 σ + 2 π)
- Strongest bond and shortest bond length
- Diamagnetic in nature
How does N2 compare with N2+ ion?
When one electron is removed from N2, the electron is lost from $\sigma 2p_z$ MO.
The configuration becomes:
$$N_2^+ : KK \, (\sigma 2s)^2 \, (\sigma^* 2s)^2 \, (\pi 2p_x)^2 \, (\pi 2p_y)^2 \, (\sigma 2p_z)^1$$
Bond order:
$$\text{Bond order} = \frac{7-2}{2} = 2.5$$
- Bond order of N2+ (2.5) is less than N2 (3).
- Therefore, bond strength decreases and bond length increases in N2+ compared to N2.
Key Takeaways:
- N2+ has lower bond order than N2
- Bond becomes weaker
- Bond length increases
What is the Molecular Orbital Theory of Oxygen Molecule (O2)?
Electronic configuration of oxygen atom:
$$1s^2 \, 2s^2 \, 2p_x^2 \, 2p_y^1 \, 2p_z^1$$
Oxygen molecule has 16 electrons.
MO configuration:
$$O_2 : KK \, (\sigma 2s)^2 \, (\sigma^* 2s)^2 \, (\sigma 2p_z)^2 \, (\pi 2p_x)^2 \, (\pi 2p_y)^2 \, (\pi^* 2p_x)^1 \, (\pi^* 2p_y)^1$$
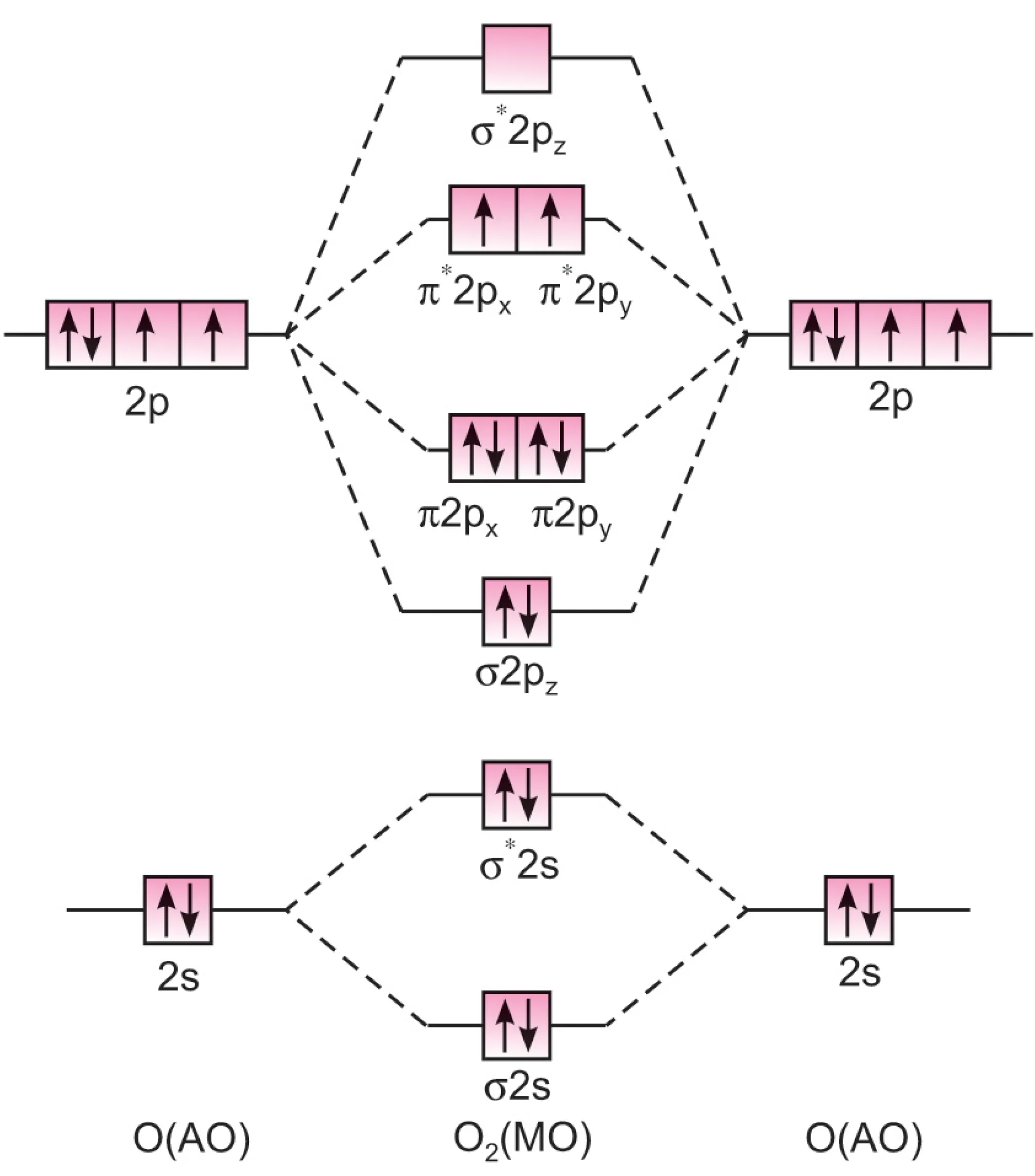
O2 molecule
Bond order:
$$\text{Bond order} = \frac{8-4}{2} = 2$$
Thus, O2 has two bonds (one $\sigma$ and one $\pi$).
- Last two electrons remain unpaired, hence O2 is paramagnetic.
- Bond dissociation energy: 498 kJ/mol
- Bond length: 121 pm
Key Takeaways:
- Bond order of O2 = 2
- Double bond present (1 σ + 1 π)
- Paramagnetic due to 2 unpaired electrons
- Matches experimental values
How does O2 compare with O2+, O2–, and O22-⁻?
O2+ Ion
Formed by removal of one electron from $\pi^* 2p_y$.
$$O_2^+ : KK \, (\sigma 2s)^2 (\sigma^* 2s)^2 (\sigma 2p_z)^2 (\pi 2p_x)^2 (\pi 2p_y)^2 (\pi^* 2p_x)^1$$
Bond order:
$$\text{Bond order} = \frac{8-3}{2} = 2.5$$
- Stronger bond than O2
- Shorter bond length
Key Takeaways:
- Bond order = 2.5
- Stronger and shorter bond than O2
O2– Ion (Superoxide)
Formed by addition of one electron in antibonding MO.
$$O_2^- : KK \, (\sigma 2s)^2 (\sigma^* 2s)^2 (\sigma 2p_z)^2 (\pi 2p_x)^2 (\pi 2p_y)^2 (\pi^* 2p_x)^2 (\pi^* 2p_y)^1$$
Bond order:
$$\text{Bond order} = \frac{8-5}{2} = 1.5$$
- Weaker bond than O2
- Bond length increases
Key Takeaways:
- Bond order = 1.5
- Bond weaker than O2
- Bond length larger
O22- Ion (Peroxide)
Formed by addition of two electrons in antibonding orbitals.
$$O_2^{2-} : KK \, (\sigma 2s)^2 (\sigma^* 2s)^2 (\sigma 2p_z)^2 (\pi 2p_x)^2 (\pi 2p_y)^2 (\pi^* 2p_x)^2 (\pi^* 2p_y)^2$$
Bond order:
$$\text{Bond order} = \frac{8-6}{2} = 1$$
- Weakest bond among all
- Longest bond length
Key Takeaways:
- Bond order = 1
- Weakest bond strength
- Longest bond length
What is the Experimental Comparison of O2 , O2+, O2–, and O22-?
| Species | Bond Order | Bond Dissociation Energy (kJ/mol) | Bond Length (pm) |
|---|---|---|---|
| O2+ | 2.5 | 625 | 112 |
| O2 | 2.0 | 495 | 121 |
| O2– | 1.5 | 395 | 130 |
| O22- | 1.0 | Weakest bond | Longest length |
Trend:
- Bond energy: $O_2^+ > O_2 > O_2^- > O_2^{2-}$
- Bond length: $O_2^{2-} > O_2^- > O_2 > O_2^+$
Key Takeaways:
- Highest bond energy in O2+
- Longest bond length in O22-
- Order matches experimental results
What is the Molecular Orbital Theory of Fluorine Molecule (F2)?
Electronic configuration of fluorine atom:
$$1s^2 \, 2s^2 \, 2p^5$$
Total valence electrons in F2 = 14.
MO configuration:
$$F_2 : KK (\sigma 2s)^2 (\sigma^* 2s)^2 (\sigma 2p_z)^2 (\pi 2p_x)^2 (\pi 2p_y)^2 (\pi^* 2p_x)^2 (\pi^* 2p_y)^2$$
Bond order:
$$\text{Bond order} = \frac{8-6}{2} = 1$$
- One $\sigma$ bond present
- All electrons paired → Diamagnetic
- Bond dissociation energy: 159 kJ/mol
- Bond length: 143 pm
Key Takeaways:
- Bond order = 1
- Single bond present
- Diamagnetic molecule
- Matches experimental values
Why does Neon Molecule (Ne2) not exist?
Electronic configuration of neon atom:
$$1s^2 \, 2s^2 \, 2p^6$$
MO configuration of Ne2 :
$$Ne_2 : KK (\sigma 2s)^2 (\sigma^* 2s)^2 (\sigma 2p_z)^2 (\pi 2p_x)^2 (\pi 2p_y)^2 (\pi^* 2p_x)^2 (\pi^* 2p_y)^2 (\sigma^* 2p_z)^2$$
Bond order:
$$\text{Bond order} = \frac{8-8}{2} = 0$$
Thus, Ne2 molecule does not exist because there is no net bond formation.
Key Takeaways:
- Bond order = 0
- No bond formation possible
- Ne₂ molecule does not exist
Complete Comparison Table of N2, O2, F2, and Ne2
Here is a summarized SEO-friendly comparison table of the four diatomic molecules based on molecular orbital theory:
| Molecule | Bond Order | Bond Type | Bond Dissociation Energy (kJ/mol) | Bond Length (pm) | Magnetic Nature | Existence |
|---|---|---|---|---|---|---|
| N2 | 3 | Triple bond (1 σ + 2 π) | 945 | 110 | Diamagnetic | Exists |
| O2 | 2 | Double bond (1 σ + 1 π) | 498 | 121 | Paramagnetic | Exists |
| F2 | 1 | Single σ bond | 159 | 143 | Diamagnetic | Exists |
| Ne2 | 0 | No bond | – | – | – | Does not exist |
Summary
- Bond order decreases from N2 → O2 → F2 → Ne2
- Bond strength trend: $N_2 > O_2 > F_2$ (Ne₂ does not form a bond).
- Bond length trend: $N_2$ (shortest) → $O_2$ → $F_2$ (longest).
- Magnetic property trend:
- N2 → Diamagnetic
- O2 → Paramagnetic
- F2 → Diamagnetic
- Ne2 → Not stable
✅ Conclusion:
- N2 is the most stable diatomic molecule due to its triple bond.
- O2 has a paramagnetic nature explained by MO theory.
- F2 has the weakest bond among the stable molecules.
- Ne2 does not exist because its bond order is zero.
Short Answer Type Questions (SAT)
Q1. Why does N2 molecule have a higher bond dissociation energy than O2 molecule?
Answer:
N2 has a bond order of 3 (triple bond), while O2 has a bond order of 2 (double bond).
- Higher bond order = stronger bond = higher bond dissociation energy.
Therefore, N2 has higher bond dissociation energy than O2.
Q2. Why is O2 paramagnetic but N2 diamagnetic?
Answer:
In O2, two unpaired electrons occupy $\pi^2p_x$ and $\pi^2p_y$ orbitals → paramagnetic.
In N2, all molecular orbitals are completely filled with paired electrons → diamagnetic.
Q3. Why does Ne2 not exist?
Answer:
Bond order of Ne2 is:
$$\text{Bond order} = \frac{8-8}{2} = 0$$
Since bond order is zero, no bond is formed. Hence, Ne2 does not exist.
Multiple Choice Questions (MCQs)
Q1. The bond order of N2 molecule is:
(a) 1
(b) 2
(c) 3
(d) 2.5
Answer: Correct Option (c)
Explanation:
$$\text{Bond order} = \frac{8-2}{2} = 3$$
Thus, N2 has a triple bond.
Q2. Which of the following species is paramagnetic?
(a) N2
(b) O2
(c) F2
(d) Ne2
Answer: Correct Option (b)
Explanation:
O2 has two unpaired electrons in antibonding orbitals → paramagnetic.
Q3. Which molecule has the weakest bond?
(a) N2
(b) O2
(c) F2
(d) Ne2
Answer: Correct Option (d)
Explanation:
Ne2 has bond order = 0 → no bond formed → weakest (nonexistent).
Assertion-Reason Type Questions
Q1.
Assertion (A): O2 molecule is paramagnetic.
Reason (R): O2 has unpaired electrons in antibonding $\pi^*$ orbitals.
(a) Both A and R are true, and R is the correct explanation of A.
(b) Both A and R are true, but R is not the correct explanation of A.
(c) A is true, R is false.
(d) A is false, R is true.
Answer: Correct Option (a)
Explanation:
O2 has two unpaired electrons in $\pi^*$ orbitals, making it paramagnetic.
Q2.
Assertion (A): Bond order of N2 is greater than bond order of O2.
Reason (R): N2 has 14 electrons while O2 has 16 electrons.
(a) Both A and R are true, and R is the correct explanation of A.
(b) Both A and R are true, but R is not the correct explanation of A.
(c) A is true, R is false.
(d) A is false, R is true.
Answer: Correct Option (b)
Explanation:
Bond order of N2 = 3, bond order of O2 = 2. This is not because of just electron count, but due to MO filling order.
Case Study Question
Case Study:
The molecular orbital theory explains the stability and magnetic properties of diatomic molecules. Consider N2, O2, F2, and Ne2 molecules.
Questions:
- Write the bond order of each molecule.
- Which of the given molecules is paramagnetic?
- Arrange the molecules in increasing order of bond length.
- Why does Ne₂ not exist?
Answers:
- Bond orders:
- N2 = 3
- O2 = 2
- F2 = 1
- Ne2 = 0
- O2 is paramagnetic due to two unpaired electrons in antibonding orbitals.
- Bond length order:
$$N_2 < O_2 < F_2 < Ne_2$$ - Ne2 has bond order = 0 → no bond formed → does not exist.
Explanation:
Bond order directly relates to bond strength and inversely to bond length. Molecules with higher bond order are shorter and stronger.


