Anand Classes explains why ice, being less dense, floats over water and why the density of water is maximum at 4°C, based on the concept of hydrogen bonding and molecular arrangement. Students will learn how the open hexagonal structure of ice formed by extensive hydrogen bonding makes it less dense than liquid water. The lesson also covers how, upon cooling, the density of water first increases and then decreases after 4°C, making 4°C the temperature of maximum density. This topic is essential for understanding anomalous behavior of water in Class 11, Class 12, JEE, and NEET Chemistry, and is supported with diagrams, solved examples, MCQs, Q&A, Assertion Reason, and Case Study questions. Click the print button to download study material and notes.
Why is Water Considered an Unusual Liquid?
Water is the most common substance on Earth’s surface, but it has many unusual properties compared to other liquids.
- Water can dissolve a wide variety of compounds: ionic compounds, polar inorganic compounds, and organic compounds.
- It shows high heat of vaporisation, high heat of fusion, high specific heat, and a wide liquid range (0°C to 100°C).
These unusual properties are explained by hydrogen bonding in water.
Key Takeaways:
- Water dissolves many substances due to its polar nature.
- Its unusual thermal properties arise from hydrogen bonding.
What is the Role of Hydrogen Bonding in Water and Ice?
The phenomenon of hydrogen bonding is responsible for the unusual behaviour of water.
- Ice is less dense than water (so it floats).
- Density of water is maximum at 4°C.
Both of these can be explained by hydrogen bonding and molecular arrangement.
Key Takeaways:
- Hydrogen bonding explains why ice floats.
- Density of water behaves anomalously, reaching maximum at 4°C.
Why is Ice Lighter than Water ? Why Ice floats over Water ?
X-ray studies reveal that in ice:
- Each oxygen atom is tetrahedrally surrounded by four hydrogen atoms.
- Two H atoms are bonded by covalent bonds. These hydrogen atoms lie closer to the oxygen atom at a distance of 1.00 Å (O–H, bond length ≈ 1.00 Å).
- Two H atoms are linked by hydrogen bonds. They lie away from oxygen atom at a distance of 1.76Å (bond length ≈ 1.76 Å).
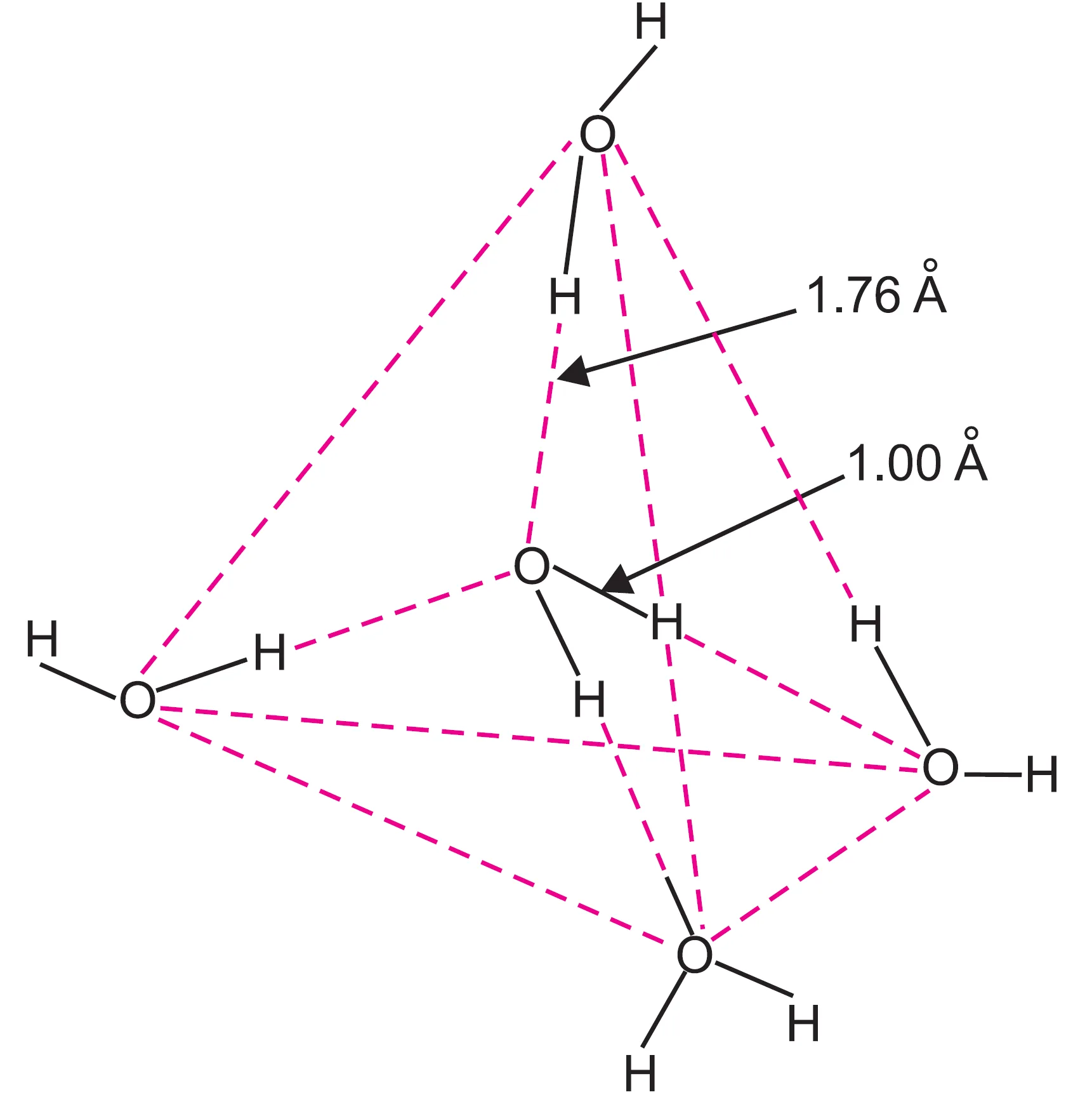
- Oxygen atoms of water molecules are located at the corners of a tetrahedron.
This creates an open cage-like structure in ice.
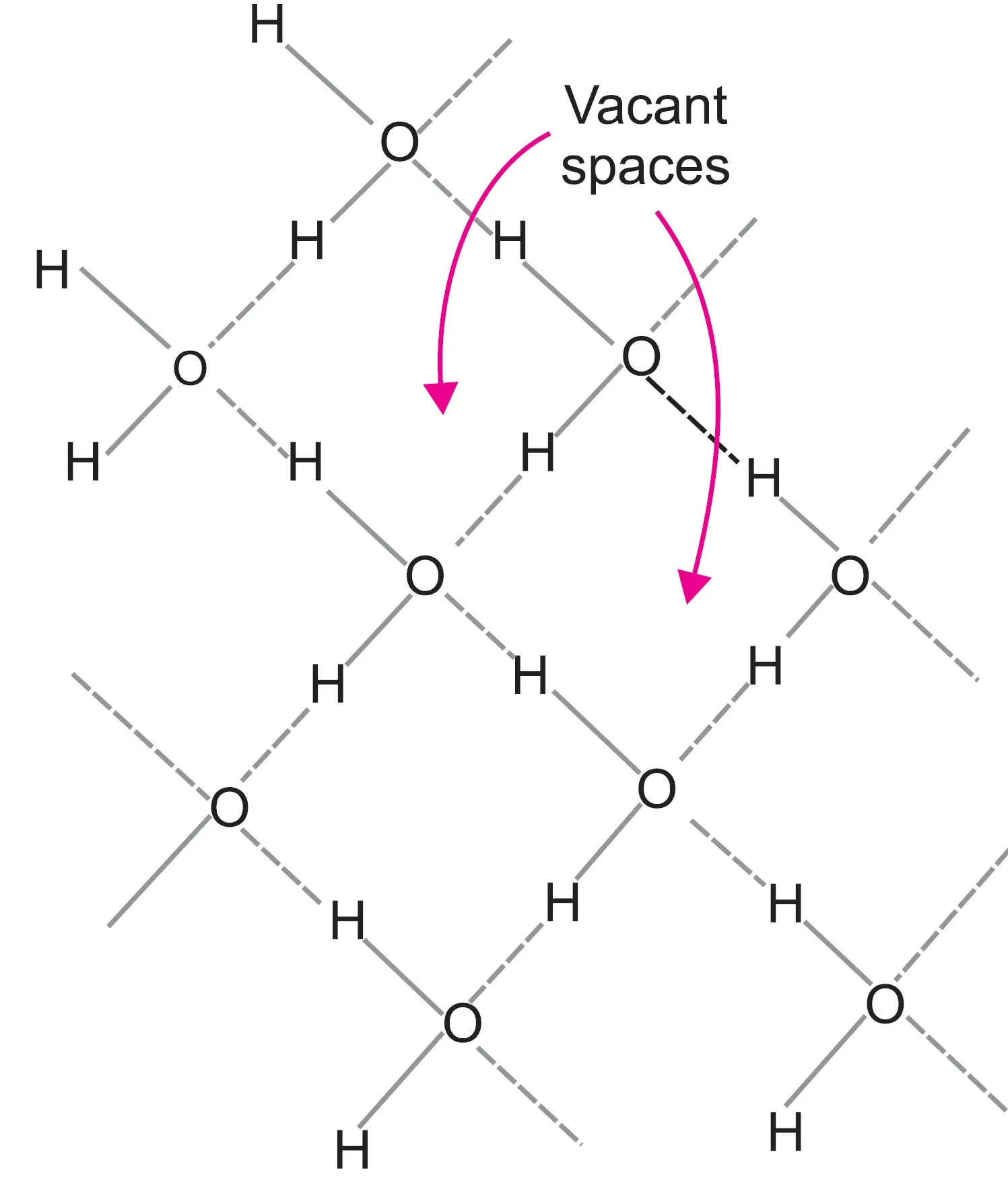
As a result of tetrahedral arrangement of H2O molecules in the solid state, the ice structure extends in three dimensions.
- Hydrogen bonds are longer than covalent bonds → water molecules are not packed closely.
- Presence of vacant spaces leads to a structure with larger volume for the same mass.
- Hence, density of ice is less than water, so ice floats on water.
Key Takeaways:
- Ice has an open cage-like structure due to hydrogen bonding.
- This explains why ice is lighter and less dense than liquid water.
Why Does Water Have Maximum Density at 4°C?
When ice melts, the open cage structure begins to collapse:
- Hydrogen bonds break partially, allowing water molecules to come closer.
- Water molecules from the ice lattice fill vacant spaces, decreasing volume.
- This increases density as temperature rises above 0°C.
At 4°C, water reaches maximum density.
- Above 4°C, kinetic energy increases, molecules move apart again.
- Expansion due to molecular motion becomes greater than contraction from hydrogen bond collapse.
- Thus, density decreases beyond 4°C.
When ice is melted by increasing its temperature, the open cage structure starts collapsing due to partial break down of the hydrogen bonds. This allows H2O molecules to come closer and also the water molecules separated from ice structure fill the vacant spaces in open-cage structure. This results into concentration of volume. Hence, the density of water starts increasing on heating above the melting point of ice and becomes maximum at 4°C.
On further rise in temperature, the kinetic energy of molecules increases
and they once again start moving away from one another. This causes expansion which is much more than the concentration caused by breaking of hydrogen bonds. Therefore, above 4°C the density of water once again starts decreasing. Thus, the maximum density of water is at 4°C.
Key Takeaways:
- Water shows anomalous expansion.
- Density is maximum at 4°C due to balance between hydrogen bonding and molecular motion.
Summary
Water is an unusual liquid because of hydrogen bonding, which gives it properties different from most other liquids:
- Solvent ability: Water dissolves many ionic and polar substances.
- Thermal properties: High heat of vaporisation, fusion, specific heat, and wide liquid range.
- Ice structure: Open tetrahedral network of hydrogen bonds makes ice less dense, so it floats on water.
- Density anomaly: Water reaches maximum density at 4°C due to structural collapse of hydrogen-bonded cages.
👉 These unique properties of water are crucial for climate regulation, aquatic life survival, and biological systems, making water the most important liquid on Earth.
Short Answer Type (SAT) Questions on Water
Q1. Why is water considered an unusual liquid?
Answer: Water has unusual properties such as high heat of vaporisation, high heat of fusion, high specific heat, wide liquid range, and maximum density at 4°C. These are due to hydrogen bonding between water molecules.
Q2. Why does ice float on water?
Answer: Ice has an open cage-like structure due to hydrogen bonding. This structure occupies larger volume with vacant spaces, making ice less dense than water. Hence, ice floats.
Q3. Why does water show maximum density at 4°C?
Answer: On melting, hydrogen bonds partially break, allowing molecules to come closer and fill vacant spaces, increasing density. At 4°C, this effect is maximum. Beyond 4°C, thermal expansion dominates, reducing density.
Q4. Why does water have a wide liquid range compared to other liquids?
Answer: Strong intermolecular hydrogen bonding in water requires higher energy to break, so it remains liquid between 0°C and 100°C, giving a wide liquid range.
Multiple Choice Questions (MCQs) on Water
Q5. Which of the following is responsible for the unusual properties of water?
(a) Ionic bonds
(b) Covalent bonds
(c) Hydrogen bonds
(d) van der Waals forces
Answer: (c) Hydrogen bonds
Explanation: Hydrogen bonding in water leads to its high boiling point, density anomaly, and solvent ability.
Q6. Why is density of ice lower than that of water?
(a) Ice molecules are lighter
(b) Ice has stronger covalent bonds
(c) Ice has an open cage-like structure due to hydrogen bonding
(d) Ice contains fewer water molecules
Answer: (c) Ice has an open cage-like structure due to hydrogen bonding
Explanation: The cage structure with empty spaces makes ice less dense.
Q7. At what temperature does water show maximum density?
(a) 0°C
(b) 2°C
(c) 4°C
(d) 100°C
Answer: (c) 4°C
Explanation: Water shows anomalous expansion, reaching maximum density at 4°C.
Q8. Which property of water is crucial for aquatic life survival in cold climates?
(a) High specific heat
(b) Ice floating on water
(c) High heat of vaporisation
(d) Solvent power
Answer: (b) Ice floating on water
Explanation: Ice forms an insulating layer on water surfaces, preventing lakes and rivers from freezing completely.
Assertion–Reason Type Questions (ARQs) on Water
Q9.
Assertion (A): Ice is lighter than water.
Reason (R): Ice has an open cage-like structure with vacant spaces due to hydrogen bonding.
Options:
(a) Both A and R are true, and R is the correct explanation of A.
(b) Both A and R are true, but R is not the correct explanation of A.
(c) A is true, R is false.
(d) A is false, R is true.
Answer: (a) Both A and R are true, and R explains A.
Q10.
Assertion (A): Water has maximum density at 4°C.
Reason (R): Above 4°C, thermal expansion becomes greater than contraction caused by hydrogen bonding collapse.
Options:
(a) Both A and R are true, and R is the correct explanation of A.
(b) Both A and R are true, but R is not the correct explanation of A.
(c) A is true, R is false.
(d) A is false, R is true.
Answer: (a) Both A and R are true, and R explains A.
Q11.
Assertion (A): Water has a wide liquid range.
Reason (R): Hydrogen bonding in water requires high energy to break, raising its boiling point.
Options:
(a) Both A and R are true, and R is the correct explanation of A.
(b) Both A and R are true, but R is not the correct explanation of A.
(c) A is true, R is false.
(d) A is false, R is true.
Answer: (a) Both A and R are true, and R explains A.
Case Study on Water – An Unusual Liquid
Read the passage and answer the questions:
Water is the most common liquid on Earth but shows unusual properties due to hydrogen bonding. Ice has a cage-like structure, making it less dense than water, so it floats. Water reaches maximum density at 4°C, an anomaly that plays a key role in aquatic life survival. Water also has high specific heat, high heat of fusion, and a wide liquid range from 0°C to 100°C.
Q12.1. Why does ice float on water?
Answer: Because its cage-like structure has vacant spaces, lowering its density.
Q12.2. Why is maximum density of water at 4°C ecologically important?
Answer: It ensures that aquatic life survives since water at 4°C remains at the bottom, while ice floats on top, insulating the water body.
Q12.3. Which property of water allows it to regulate climate?
Answer: High specific heat capacity, as it absorbs and releases large amounts of heat without big temperature changes.
Q12.4. What structural feature of ice is revealed by X-ray studies?
Answer: Each oxygen atom is tetrahedrally surrounded by 4 H atoms, with 2 covalently bonded and 2 hydrogen-bonded, forming an open 3D network.


