Anand Classes explains sp³ Hybridisation with detailed study material covering the molecular formation of Methane (CH₄), Ammonia (NH₃), and Water (H₂O). Students will understand how four equivalent sp³ hybrid orbitals are formed, their orientation in space, and how they result in tetrahedral, pyramidal, and bent geometries. Clear diagrams, solved examples, MCQs, Q&A, Assertion-Reason, and case study-based questions are included for Class 11, Class 12, JEE, and NEET exam preparation. Click the print button to download study material and notes.
What is sp3 Hybridisation?
In sp3 hybridisation, one s and three p–orbitals hybridise to form four sp3 hybrid orbitals. The four sp3 hybrid orbitals will be directed towards the four corners of a regular tetrahedron and make an angle of 109° 28′ (or 109.5°) as shown in Figure below.
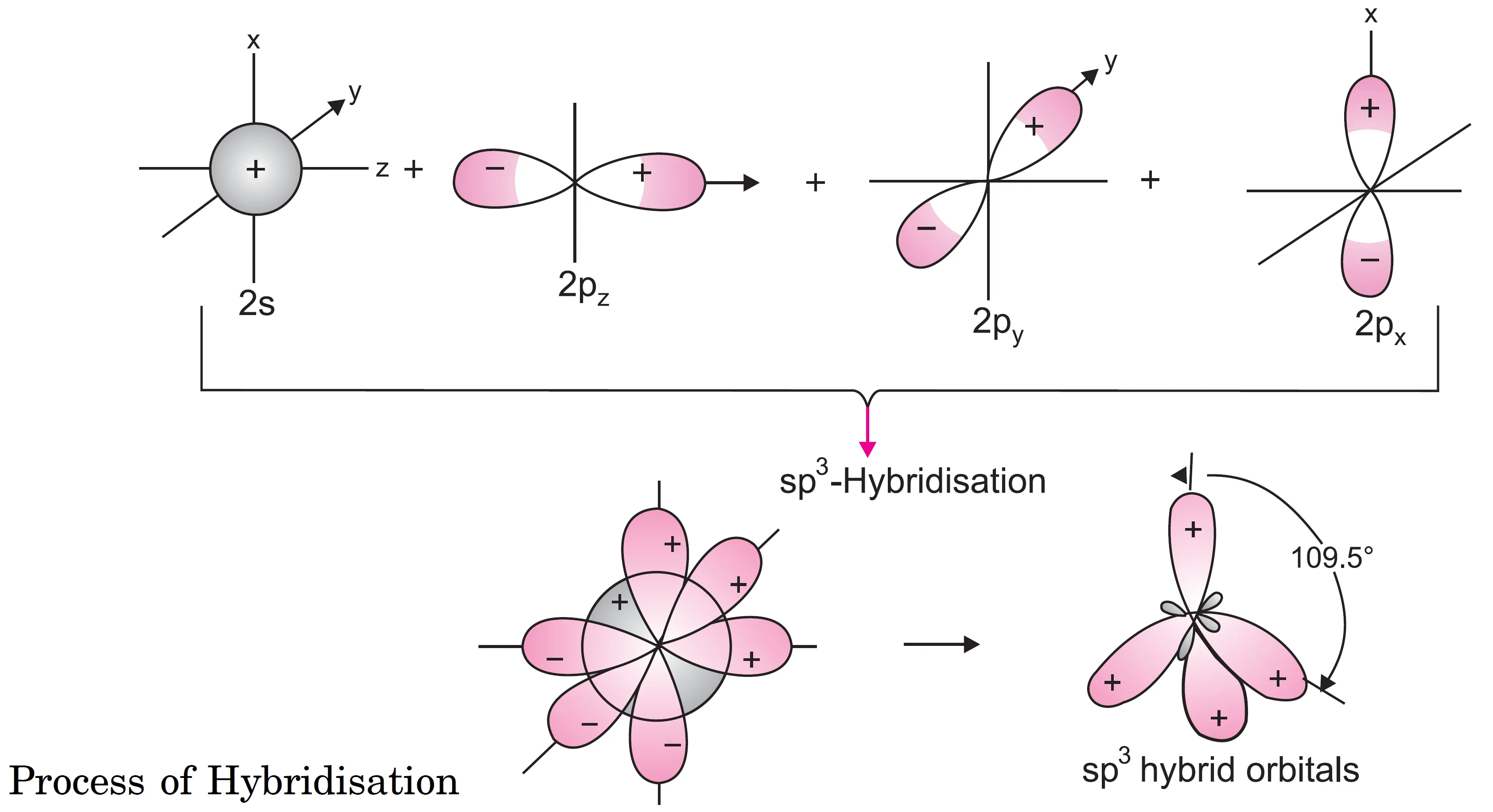
Each sp3 hybrid orbital has one fourth (25%) s–character and three–fourth (75%) p–character..
This can be illustrated by taking the example of methane (CH4) molecule.
How Does Methane (CH4) Show sp3 Hybridisation?
Formation of methane (CH4):
The central atom carbon has the ground state electronic configuration as 1s2 2s2 2px1 2py1. It has only two unpaired electrons. To account for tetravalency, one of the 2s–electrons is promoted to vacant 2p-orbital. Therefore, the excited state of carbon has four unpaired electrons.
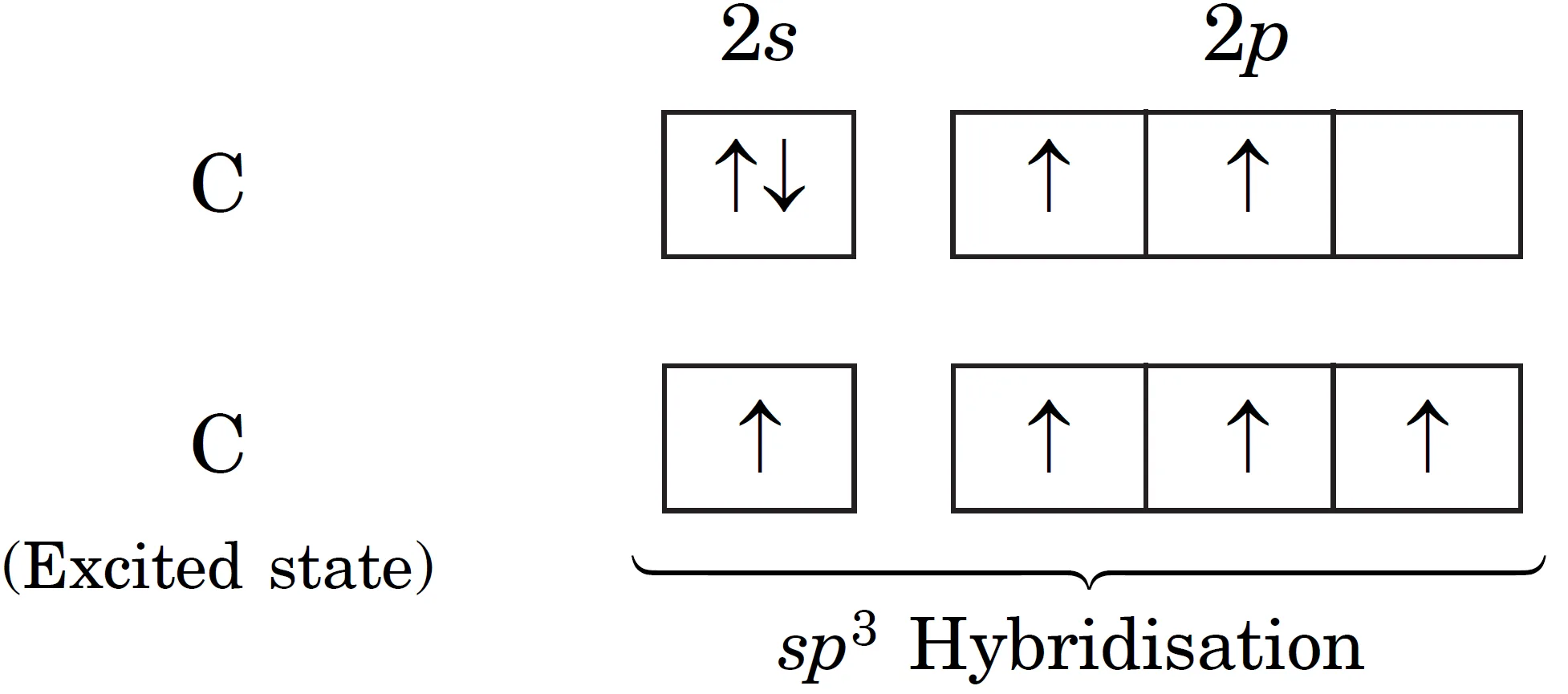
These four orbitals (one 2s and three 2p) hybridise to form four sp3 hybrid orbitals, which are oriented in tetrahedral arrangement as shown in Figure below.
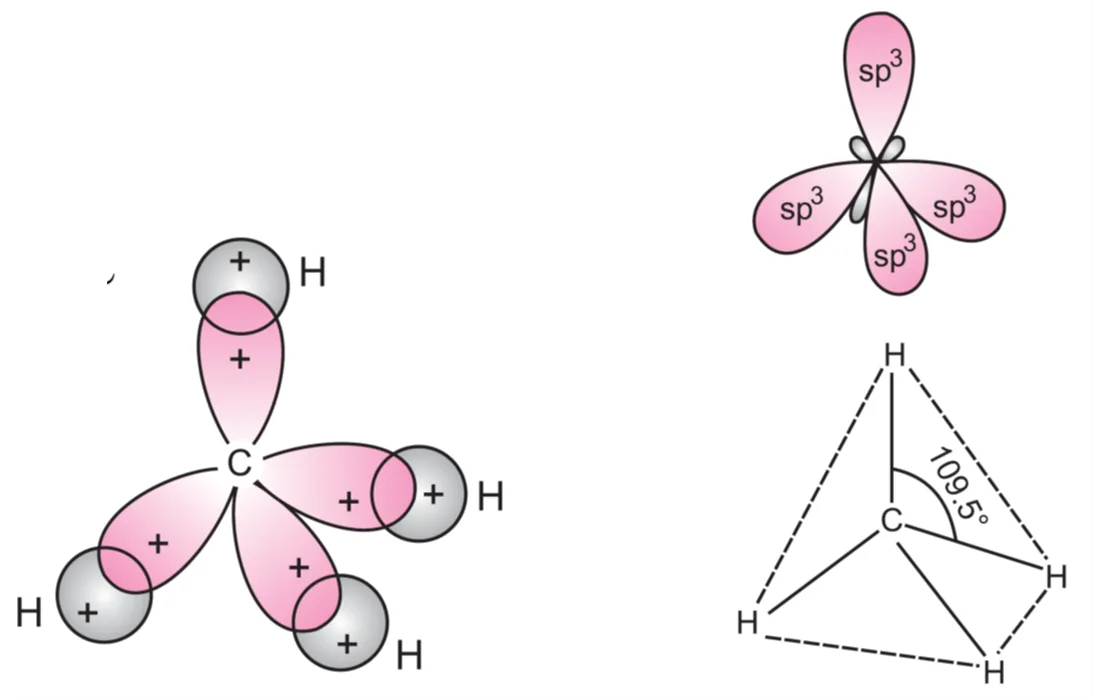
Each of these sp3 hybrid orbitals overlaps with 1s orbital of hydrogen to form four C–H bonds. Thus, methane molecule has tetrahedral geometry with each H–C–H bond angle of 109.5° as shown in Figure above.
How Does Ammonia (NH3) Exhibit sp3 Hybridisation?
Ammonia molecule (NH3):
The ground state electronic configuration of nitrogen atom is 1s2 2s2 2px1 2py1 2pz1. It contains three unpaired electrons in its ground state. Since nitrogen forms only three covalent bonds, there is no need of promotion. These four orbitals (2s2 2px1 2py1 2pz1) are hybridised to form four sp3 hybrid orbitals.
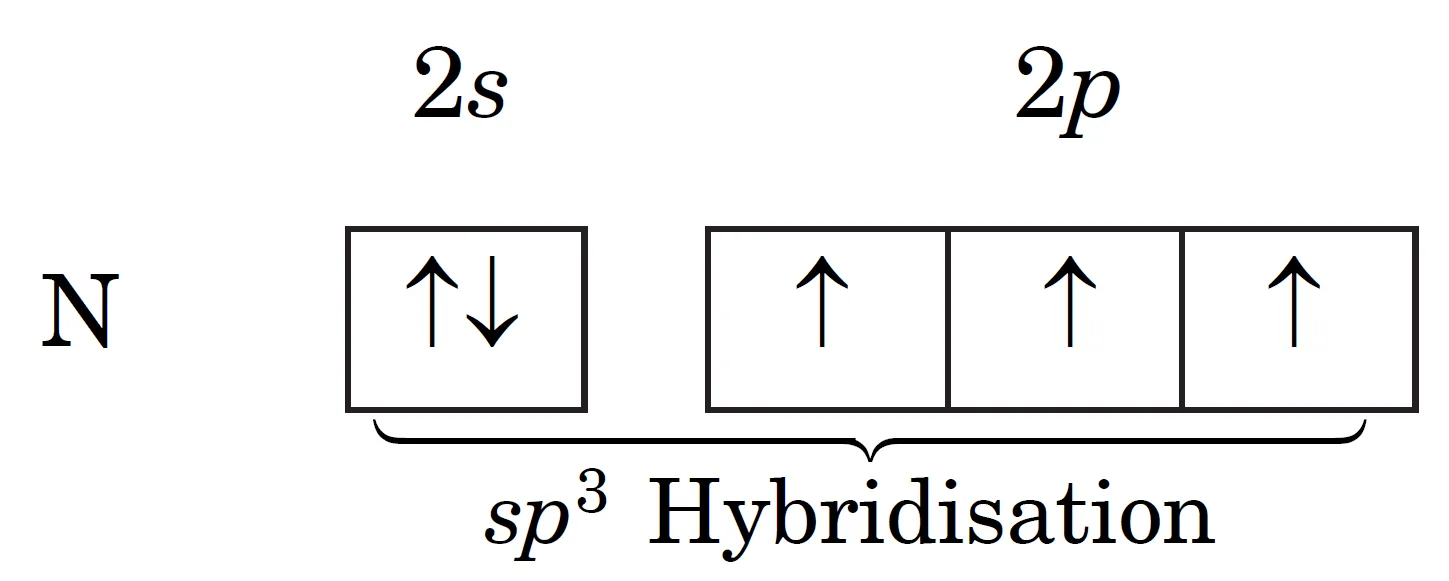
These four sp3 hybrid orbitals adopt tetrahedral arrangement. One hybrid orbital contains a lone pair (2s2) of electrons (non bonding pair of electrons) while the remaining three contain one unpaired electron each (2px1 2py1 2pz1). These three unpaired electrons (2px1 2py1 2pz1) form hybrid orbitals and overlap with 1s–orbitals of three hydrogen atoms to form three N—H sigma bonds.
Thus, in ammonia, the central atom N, is surrounded by three bond pairs (N—H bonds) and one lone pair of electrons.
We know that the force of repulsion between a lone pair and a bond pair is greater than the force of repulsion between two bond pairs of electrons. The molecule, therefore, gets a little distorted and the bond angle H—N—H decreases from 109.5° to 107°.
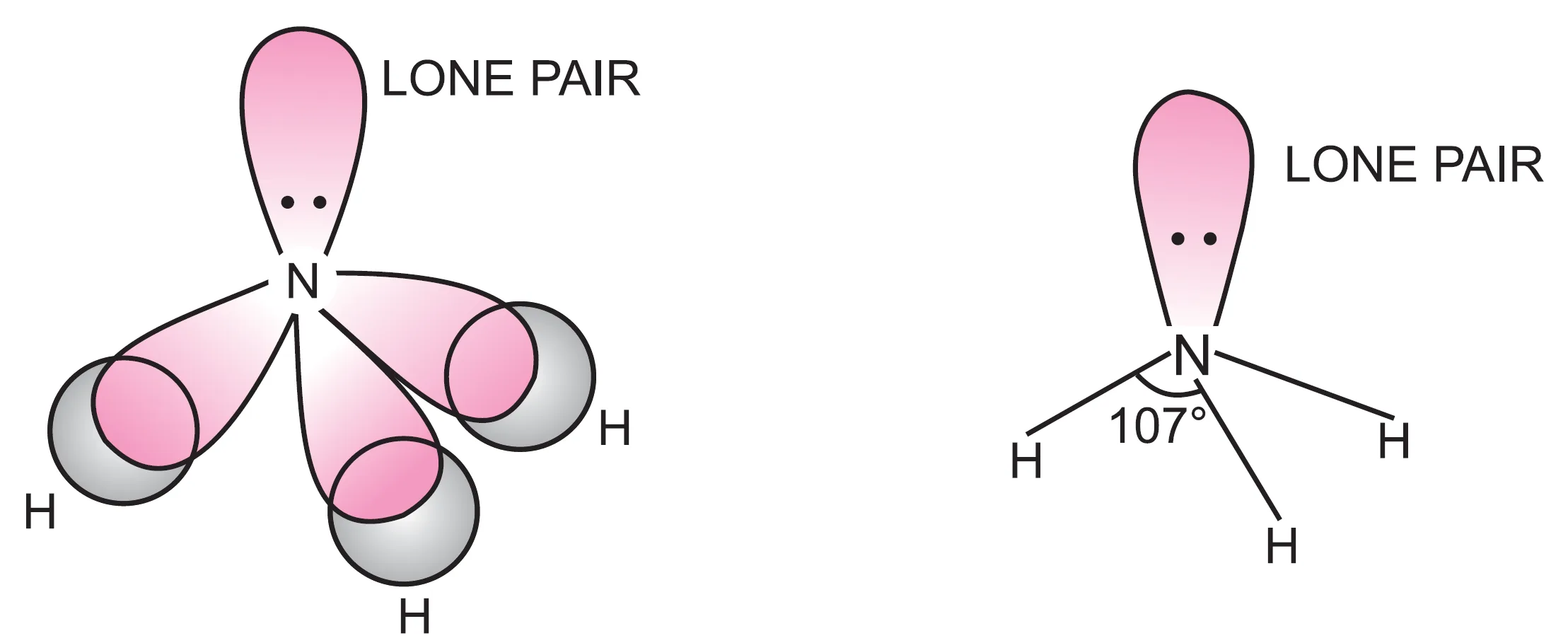
The shape of the molecule is pyramidal in which the nitrogen atom lies at the centre, three hydrogens form the base and the lone pair of electrons forms the apex of the pyramid.
How Does Water (H2O) Exhibit sp3 Hybridisation?
Formation of Water molecule (H2O):
The ground state electronic configuration of the central oxygen atom is 1s2 2s2 2px2 2py1 2pz1 and is represented in Figure.
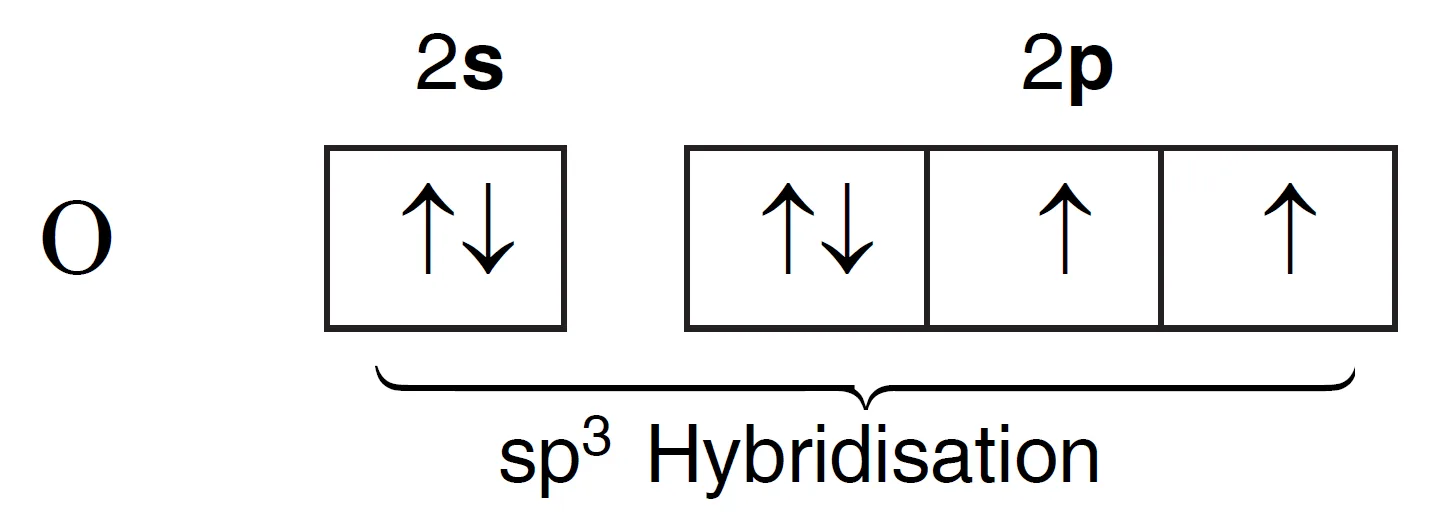
Thus, there are two unpaired electrons which can form two bonds with hydrogen atoms, without involving any excitation of the oxygen atom. These four orbitals (2s2 2px2 2py1 2pz1) undergo hybridisation to give four sp3 hybrid orbitals of equivalent energy.
These four sp3 hybrid orbitals adopt a tetrahedral structure with two corners occupied by hydrogen atoms and two corners occupied by lone pairs of electrons as shown in the figure.
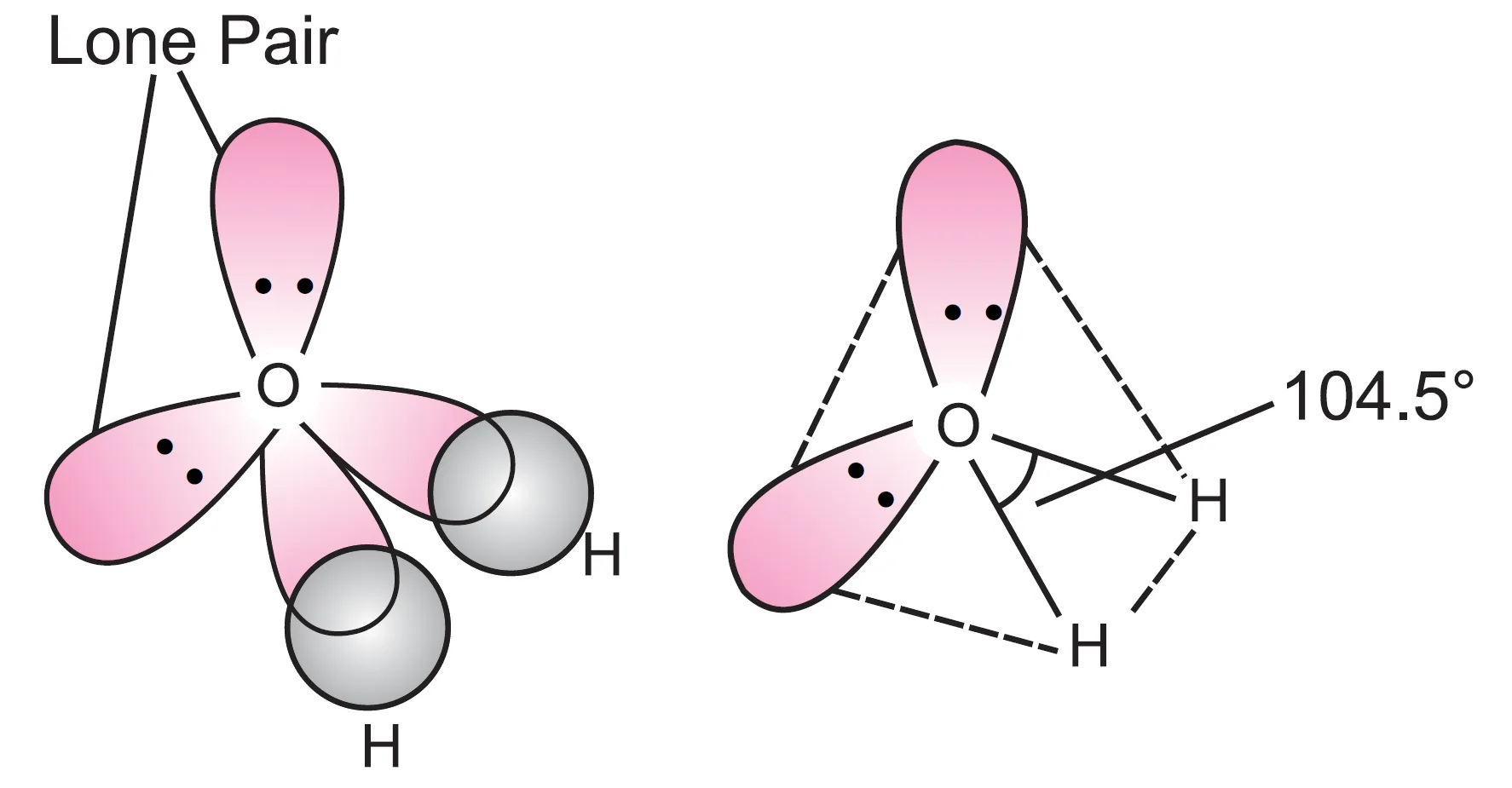
Now, we know that the force of repulsion between the two lone pairs of electrons amongst themselves is more than the force of repulsion between a lone pair and a bond pair, which in turn is more than the force of repulsion between two bond pairs of electrons. Therefore, there is greater distortion in this molecule than in ammonia.
Consequently, the bond angle H–O–H in water is 104.5°, which is even smaller than the bond angle H–N–H in ammonia. Such a structure is described as V–shaped or angular.
How Does Ethane (C2H6) Exhibit sp3 Hybridisation?
Formation of ethane:
In ethane (H3C—CH3) molecule, each carbon atom undergoes sp3 hybridisation. One of the four sp3 hybrid orbitals of one carbon atom overlaps axially with similar orbital of the other carbon atom to form a C—C sigma bond. The remaining three hybrid orbitals belonging to both the carbon atoms overlap axially with the half-filled orbitals of hydrogen atoms to form C—H sigma bonds as shown in Figure. The hybridisation is, therefore, known as tetrahedral hybridisation with bond angle 109.5o.
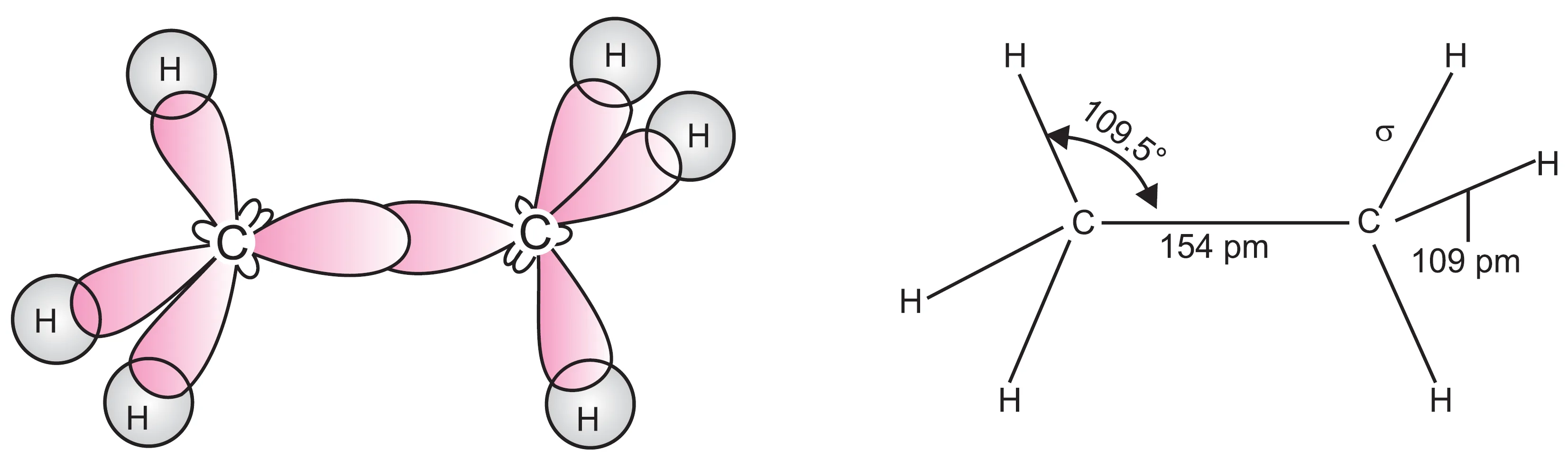
Thus, in ethane, C—C bond length is 154 pm and each C—H bond length is 109 pm.
Short Answer Conceptual Types (SAT)
Q1. What is sp³ hybridisation?
Answer: In sp³ hybridisation, one s orbital and three p orbitals mix to form four equivalent sp³ hybrid orbitals oriented towards the corners of a tetrahedron with bond angle 109.5°.
Q2. Why does carbon promote one 2s electron before hybridisation in methane?
Answer: In ground state, carbon has only two unpaired electrons. To form four bonds (tetravalency), one 2s electron is promoted to a vacant 2p orbital, giving four unpaired electrons.
Q3. What is the shape and bond angle of CH₄ according to sp³ hybridisation?
Answer: Tetrahedral shape with bond angle 109.5°.
Q4. Why does the H–N–H bond angle in NH₃ reduce to 107°?
Answer: Because of lone pair–bond pair repulsion, which is greater than bond pair–bond pair repulsion.
Q5. Why is the bond angle in H₂O (104.5°) smaller than that in NH₃ (107°)?
Answer: Because H₂O has two lone pairs causing greater lone pair–lone pair repulsion, leading to a smaller bond angle.
Multiple Choice Questions (MCQs)
Q1. The bond angle in methane (CH₄) is:
(a) 120°
(b) 180°
(c) 107°
(d) 109.5°
Answer: (d) 109.5°
Explanation: sp³ hybrid orbitals are tetrahedrally arranged, giving a bond angle of 109.5°.
Q2. The shape of ammonia (NH₃) is:
(a) Tetrahedral
(b) Pyramidal
(c) Angular
(d) Planar
Answer: (b) Pyramidal
Explanation: sp³ hybridisation with one lone pair distorts the tetrahedral arrangement to pyramidal.
Q3. The number of lone pairs present on the central atom in H₂O is:
(a) Zero
(b) One
(c) Two
(d) Three
Answer: (c) Two
Explanation: Oxygen in H₂O has two lone pairs in sp³ hybrid orbitals.
Q4. Which of the following molecules has V–shaped geometry?
(a) CH₄
(b) NH₃
(c) H₂O
(d) C₂H₆
Answer: (c) H₂O
Explanation: Two bond pairs and two lone pairs in sp³ hybridisation lead to angular or V–shaped geometry.
Q5. In ethane (C₂H₆), the C–C bond is formed by:
(a) p–p overlap
(b) s–p overlap
(c) sp³–sp³ overlap
(d) sp²–sp² overlap
Answer: (c) sp³–sp³ overlap
Explanation: Each carbon in ethane is sp³ hybridised, forming a sigma bond through sp³–sp³ overlap.
Assertion–Reason Type Questions
Q1.
Assertion (A): The bond angle in NH₃ is 107°.
Reason (R): Lone pair–bond pair repulsion is greater than bond pair–bond pair repulsion.
- (a) Both A and R are true, and R is the correct explanation of A.
- (b) Both A and R are true, but R is not the correct explanation of A.
- (c) A is true, R is false.
- (d) A is false, R is true.
Answer: (a) Both A and R are true, and R is the correct explanation of A.
Explanation: The distortion in NH₃ from ideal 109.5° to 107° is due to stronger lone pair–bond pair repulsion.
Q2.
Assertion (A): Water molecule has a bond angle of 104.5°.
Reason (R): It has two bond pairs and two lone pairs which increase lone pair–lone pair repulsion.
- (a) Both A and R are true, and R is the correct explanation of A.
- (b) Both A and R are true, but R is not the correct explanation of A.
- (c) A is true, R is false.
- (d) A is false, R is true.
Answer: (a) Both A and R are true, and R is the correct explanation of A.
Explanation: Two lone pairs cause stronger repulsion, decreasing bond angle below NH₃.
Q3.
Assertion (A): All four C–H bonds in methane are equivalent.
Reason (R): In methane, carbon undergoes sp³ hybridisation.
- (a) Both A and R are true, and R is the correct explanation of A.
- (b) Both A and R are true, but R is not the correct explanation of A.
- (c) A is true, R is false.
- (d) A is false, R is true.
Answer: (a) Both A and R are true, and R is the correct explanation of A.
Explanation: sp³ hybridisation produces four equivalent hybrid orbitals, forming four equivalent C–H bonds.
Case Study
Passage:
Methane, ammonia, and water molecules are classical examples of sp³ hybridisation. In methane (CH₄), carbon promotes one 2s electron and undergoes sp³ hybridisation to form four equivalent orbitals arranged tetrahedrally (109.5°). In ammonia (NH₃), nitrogen hybridises its valence orbitals to form four sp³ orbitals, but due to one lone pair, the bond angle reduces to 107°, giving pyramidal shape. In water (H₂O), oxygen forms four sp³ hybrid orbitals, two of which contain lone pairs. The strong lone pair–lone pair repulsions reduce bond angle to 104.5°, giving a V–shaped structure. In ethane (C₂H₆), each carbon atom undergoes sp³ hybridisation, forming a C–C sigma bond and six C–H sigma bonds.
Questions:
Q1. What is the bond angle in methane and why?
Answer: 109.5°, due to tetrahedral arrangement of sp³ orbitals.
Q2. Why does ammonia have a pyramidal shape instead of tetrahedral?
Answer: Presence of one lone pair causes repulsion, reducing bond angle to 107°, giving pyramidal shape.
Q3. Why is the bond angle in water less than in ammonia?
Answer: Water has two lone pairs, leading to greater repulsion, hence bond angle reduces to 104.5°.
Q4. What type of overlap forms the C–C bond in ethane?
Answer: sp³–sp³ sigma overlap.
Q5. How many equivalent C–H bonds are present in methane?
Answer: Four equivalent C–H sigma bonds.


