Anand Classes provides complete Class 11 Chemistry notes on the Shapes of SF₄, ClF₃, XeF₂, BrF₅, and XeF₄ Molecules explained through VSEPR Theory. Learn how lone pairs and bond pairs determine the molecular geometry of these compounds: SF₄ (see-saw), ClF₃ (T-shaped), XeF₂ (linear), BrF₅ (square pyramidal), and XeF₄ (square planar). These notes also include diagrams, solved Q&A, MCQs, assertion-reason questions, and case study-based problems to prepare effectively for NEET, JEE, and CBSE board exams. Click the print button to download study material and notes.
What is the Shape of Molecules Containing Five Electron Pairs (AB5, AB4L, AB3L2, AB2L3) ?
When the central atom is surrounded by five electron pairs, the geometry is trigonal bipyramidal. However, if one or more bond pairs are replaced by lone pairs, the geometry gets distorted.
This may be illustrated by the following examples:
(a) Molecules containing 4 bond pairs and 1 lone pair, e.g., $SF_{4}$
(b) Molecules containing 3 bond pairs and 2 lone pairs, e.g., $ClF_{3}$
(c) Molecules containing 2 bond pairs and 3 lone pairs, e.g., $XeF_{2}$
What is the Shape of SF4 (Sulphur Tetrafluoride) Molecule?
Sulphur atom ($Z = 16 : 3s^{2} 3p^{4}$) has six valence electrons. In the formation of $SF_{4}$, four electrons form four bond pairs and two electrons remain as one lone pair.
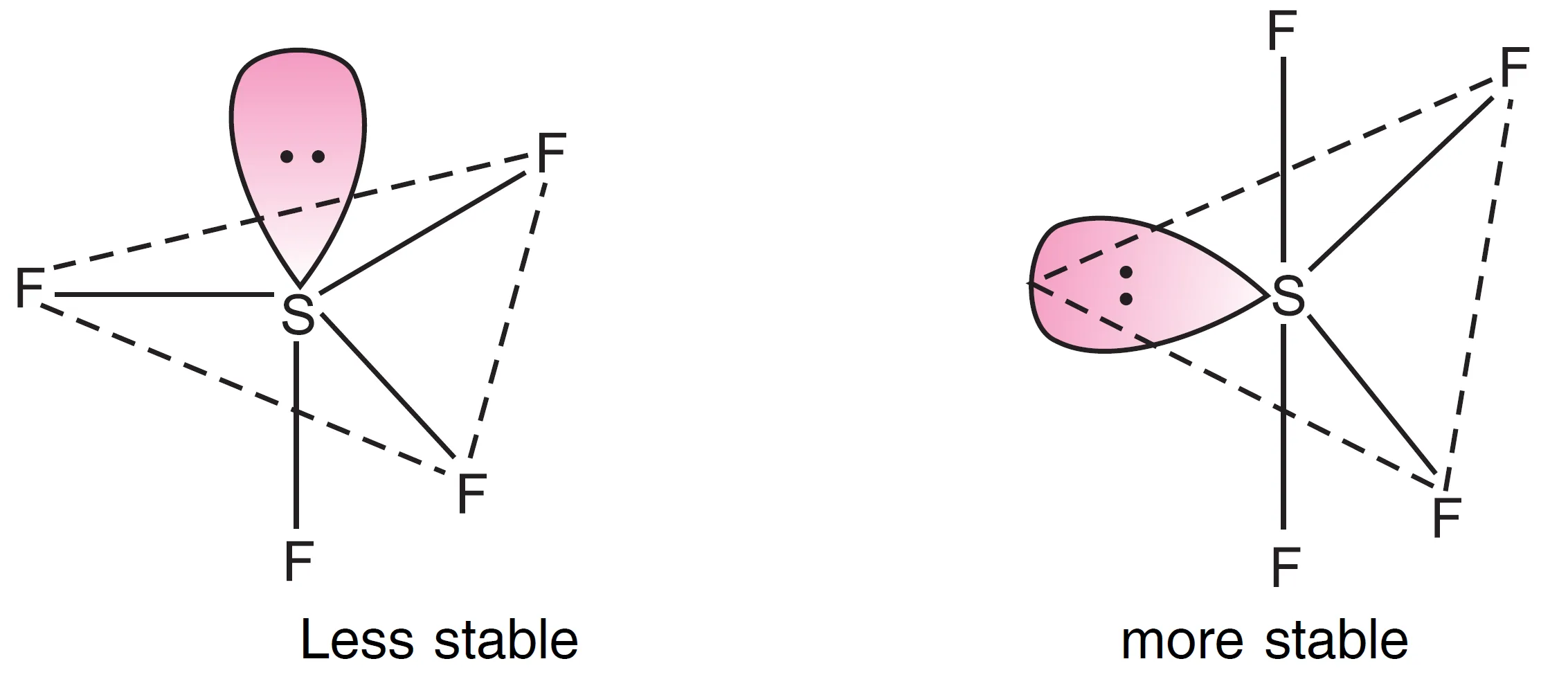
Thus, five electron pairs around sulphur adopt trigonal bipyramidal geometry in which one position is occupied by a lone pair.
- If the lone pair is at an axial position, there are 3 lp–bp repulsions at $90^{\circ}$, (see Fig.a).
- If the lone pair is at an equatorial position, there are only 2 lp–bp repulsions (see Fig.b).
Therefore arrangement (b) will experience lesser repulsions and will be stable in comparison to arrangement (a). Hence, the equatorial arrangement is more stable.
The shape of $SF_{4}$ is described as a distorted tetrahedron (folded square or `see-saw geometry).
The bond angles are $89^{circ}$ and $117^{circ}$ instead of $90^{circ}$ and $120^{circ}$.
What is the Shape of ClF3 (Chlorine Trifluoride) Molecule?
The central chlorine atom ($Z = 17 : 3s^{2} 3p^{5}$) has seven valence electrons. In the formation of $ClF_{3}$, three electrons form three bond pairs and four electrons remain as two lone pairs.
Thus, five electron pairs adopt trigonal bipyramidal geometry, in which two positions are occupied by lone pairs. Since lone pairs experience more repulsion at axial positions, both lone pairs occupy equatorial positions.
- The molecule becomes T-shaped with bond angle $87.6^{\circ}$ (instead of $90^{\circ}$).
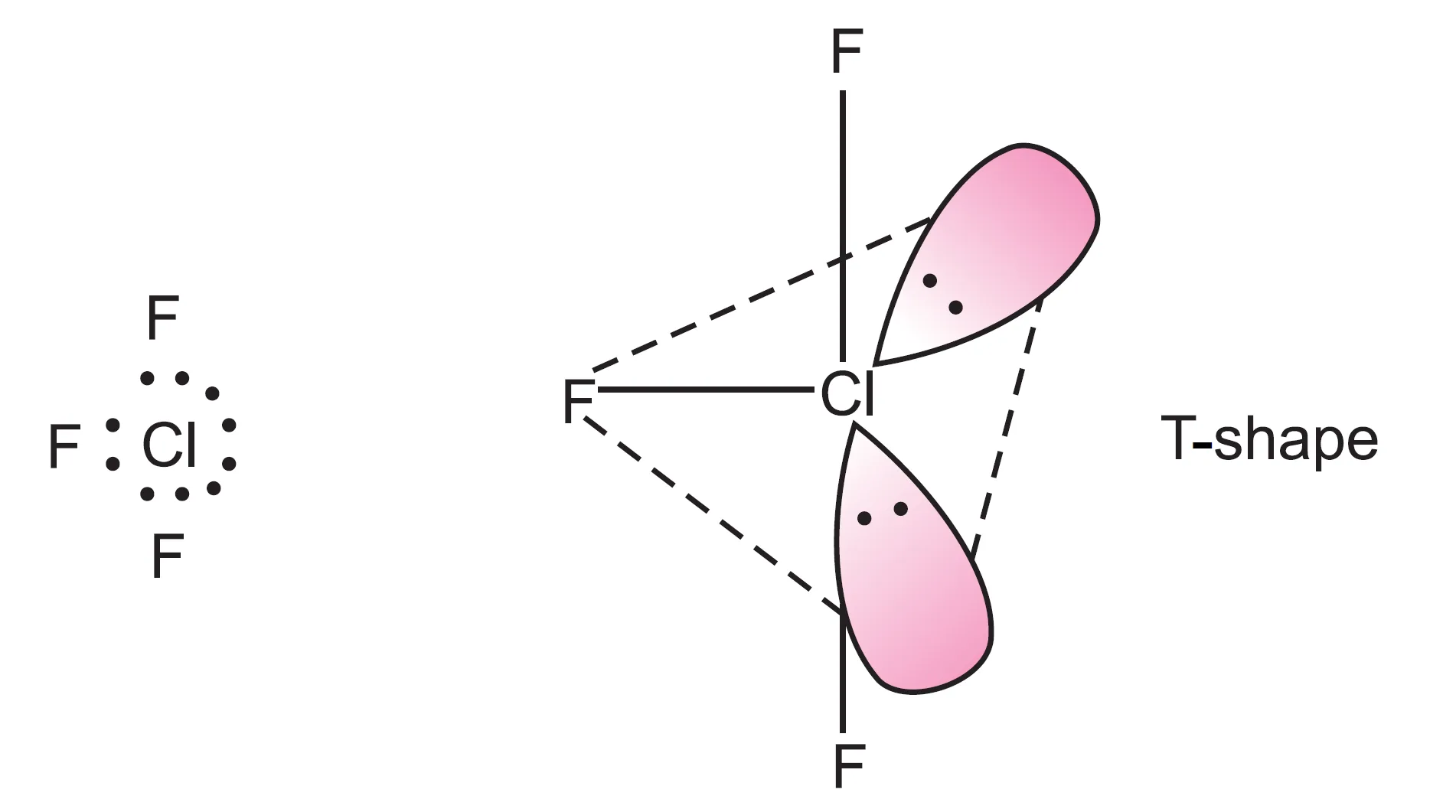
VSEPR theory (T-shaped)
Key Note:
For two lone pairs in ClF3 molecule in trigonal bipyramidal geometry, there are 3 possible arrangements:
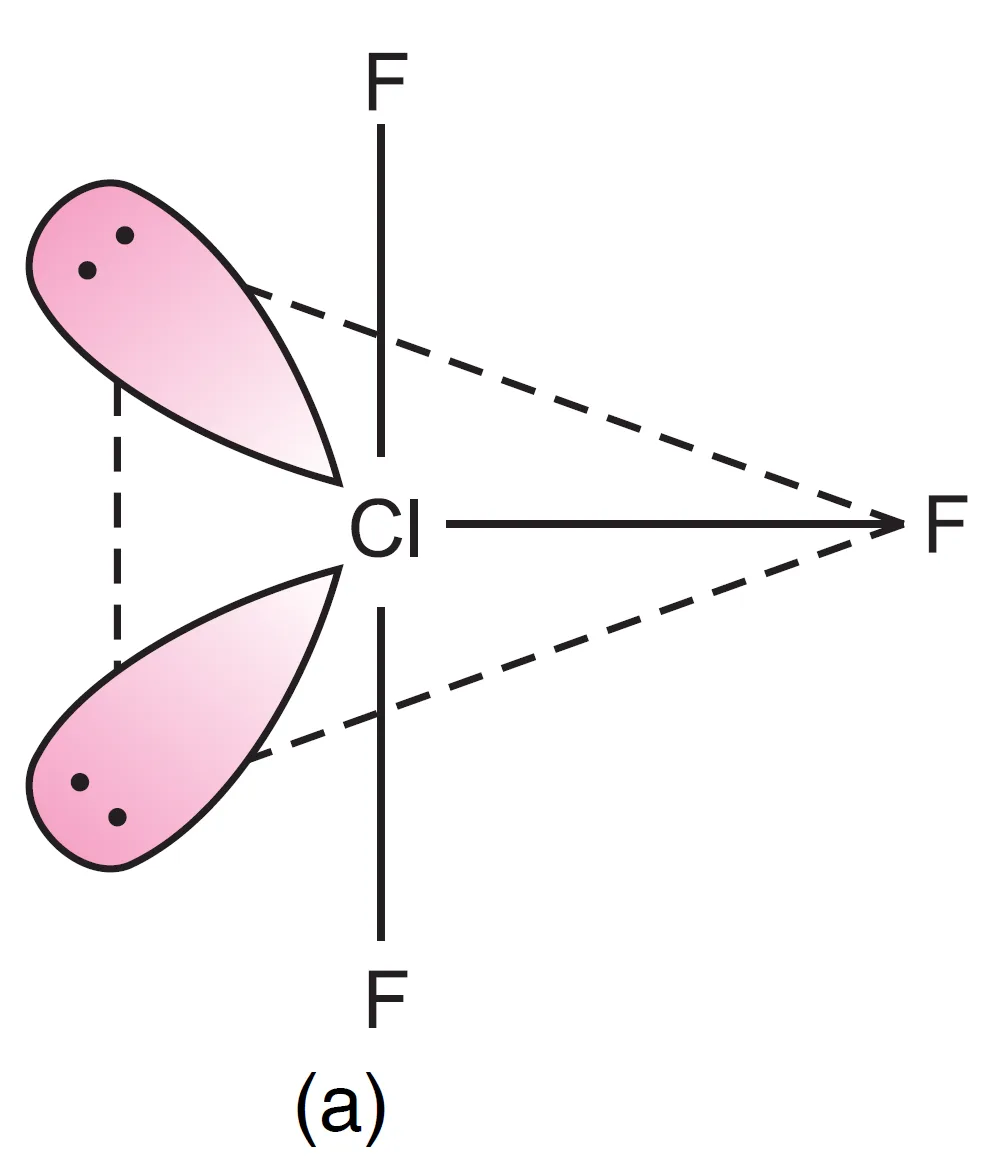
- Both lone pairs at equatorial positions (Repulsions at 90°) → lp–lp = 0, lp–bp = 4, bp–bp = 2
- One lone pair axial, one equatorial (Repulsions at 90°) → lp–lp = 1, lp–bp = 3, bp–bp = 2
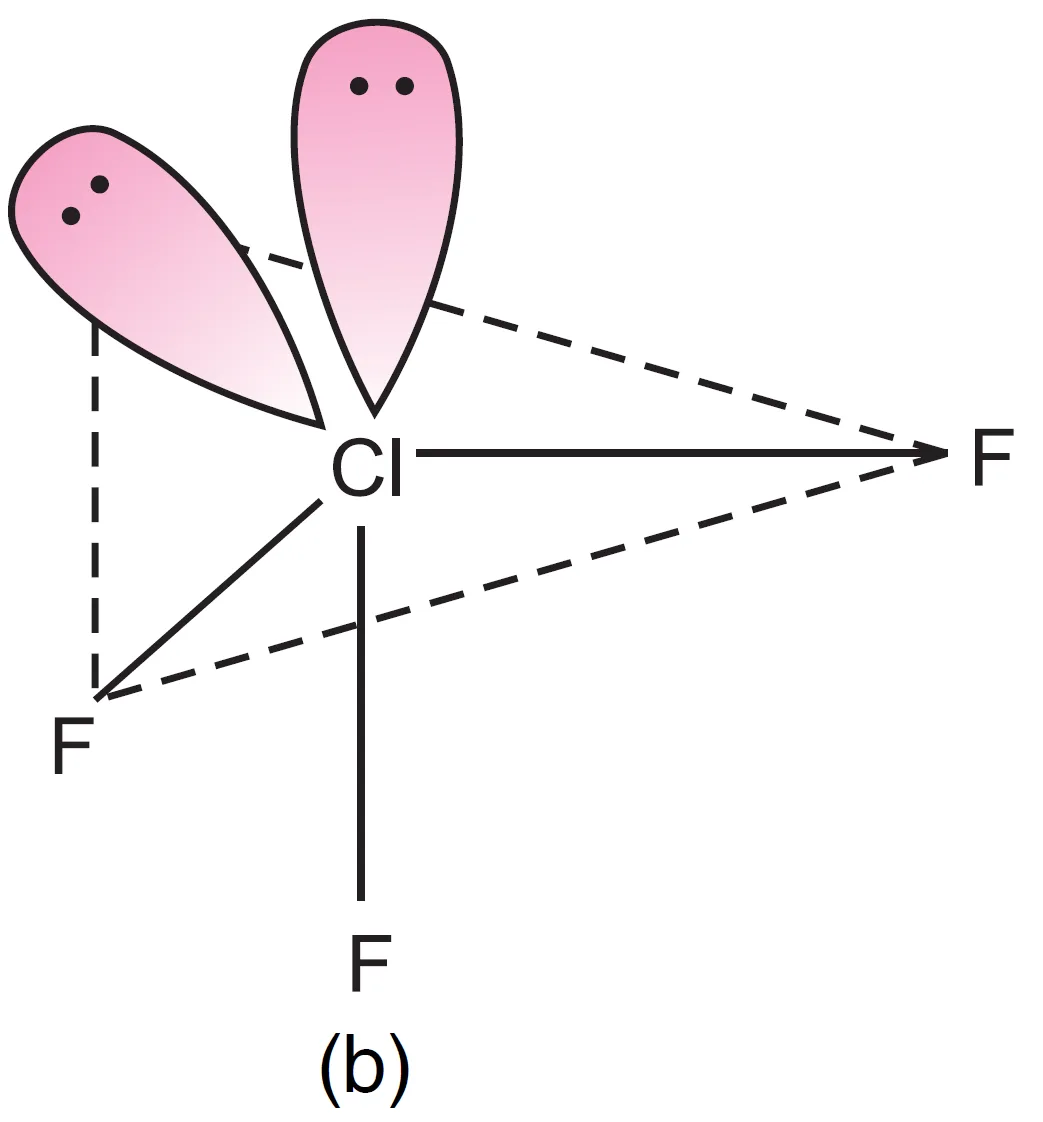
- Both lone pairs axial (Repulsions at 90°) → lp–lp = 0, lp–bp = 6, bp–bp = 0
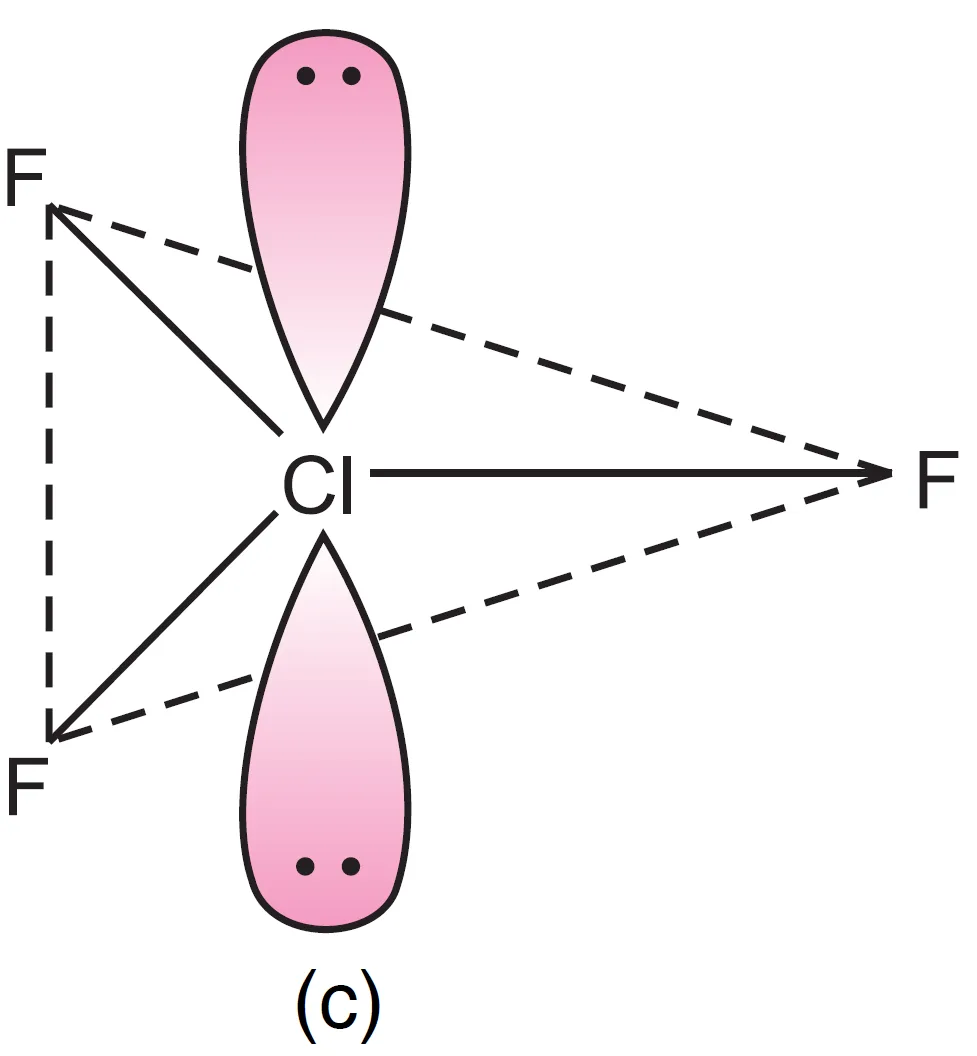
It can be seen that structure, (a) appears to be most stable because in comparison to structure (b), it has no strong lp – lp repulsion and in comparison to structure (c), it has lesser number of lp – bp repulsions.
The first arrangement (both at equatorial positions) is most stable because it minimizes strong lp–lp and lp–bp repulsions.
What is the Shape of XeF2 (Xenon Difluoride) Molecule?
Xenon atom ($Z = 54 : 5s^{2} 5p^{6}$) has eight valence electrons. In the formation of $XeF_{2}$, two electrons form two bond pairs and six electrons remain as three lone pairs.
Thus, five electron pairs adopt trigonal bipyramidal geometry. The three lone pairs occupy equatorial positions, forming the corners of an equilateral triangle. This arrangement cancels their repulsions.
The resulting geometry of $XeF_{2}$ is linear.
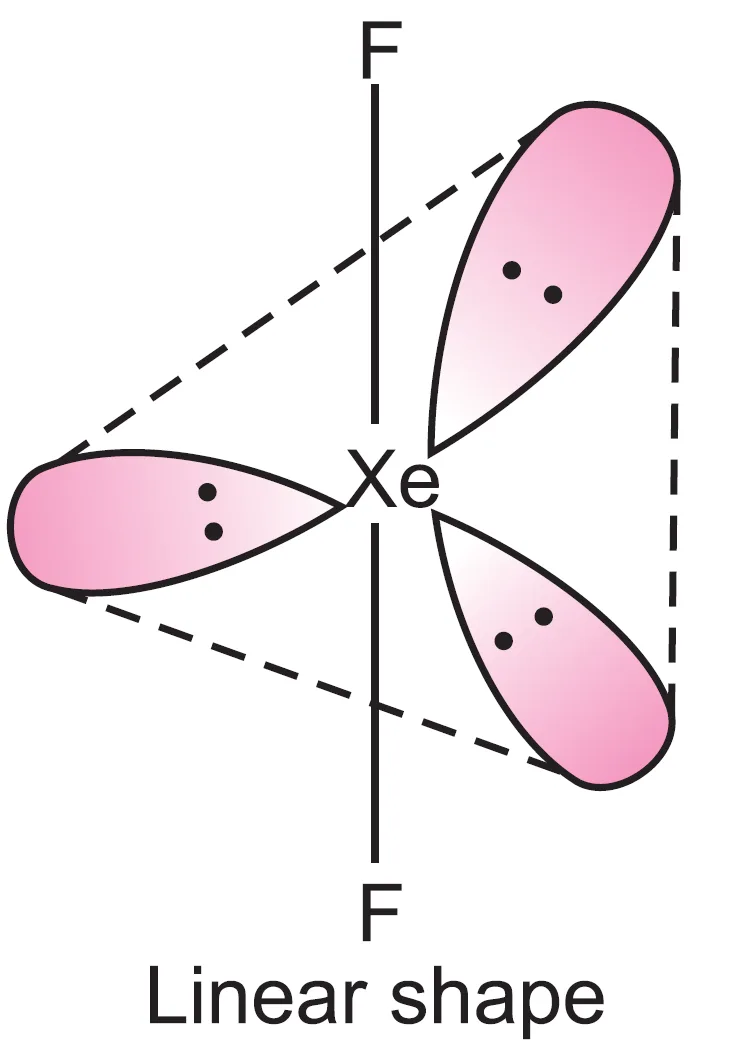
What is the Shape of Molecules Containing Six Electron Pairs (AB6, AB5L, AB4L2)?
When the central atom is surrounded by six electron pairs, the geometry is octahedral. If lone pairs are present in addition to bond pairs, the geometry gets distorted.
Examples:
(a) Molecules containing 5 bond pairs and 1 lone pair, e.g., $BrF_{5}$
(b) Molecules containing 4 bond pairs and 2 lone pairs, e.g., $XeF_{4}$
What is the Shape of BrF5 (Bromine Pentafluoride) Molecule?
The central bromine atom ($Z = 35 : 4s^{2} 4p^{5}$) has seven valence electrons. In $BrF_{5}$, five electrons form five bond pairs, and two electrons remain as one lone pair.
Thus, six electron pairs adopt octahedral geometry. Since all six positions in octahedral geometry are equivalent, the lone pair may occupy any one of them. The resulting geometry is square pyramidal.
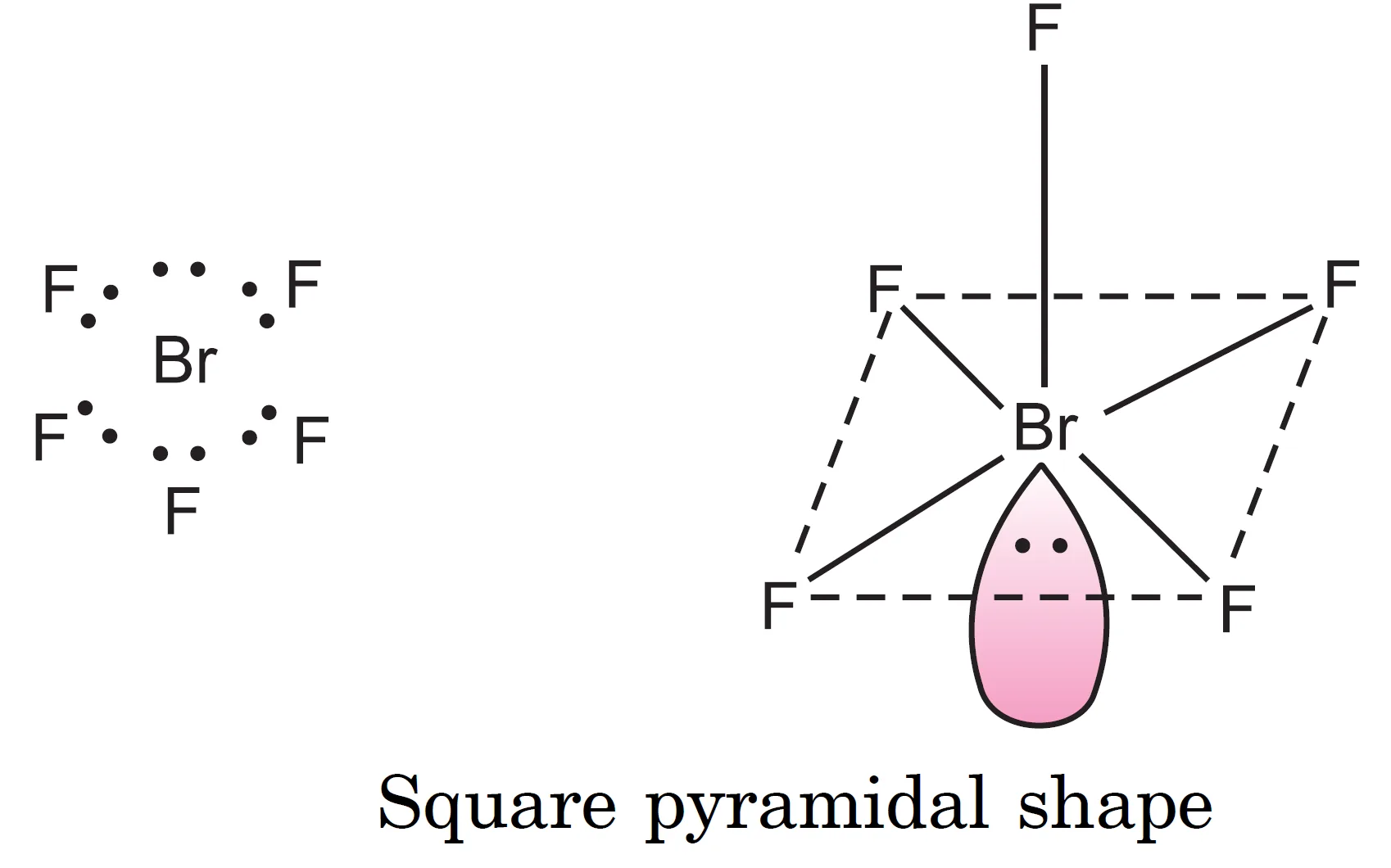
$IF_{5}$ has the same geometry.
What is the Shape of XeF4 (Xenon Tetrafluoride) Molecule?
The central xenon atom has eight valence electrons. In $XeF_{4}$, four bond pairs are formed, leaving four electrons as two lone pairs.
Thus, six electron pairs adopt octahedral geometry, with two positions occupied by lone pairs.
The resulting structure is square planar.
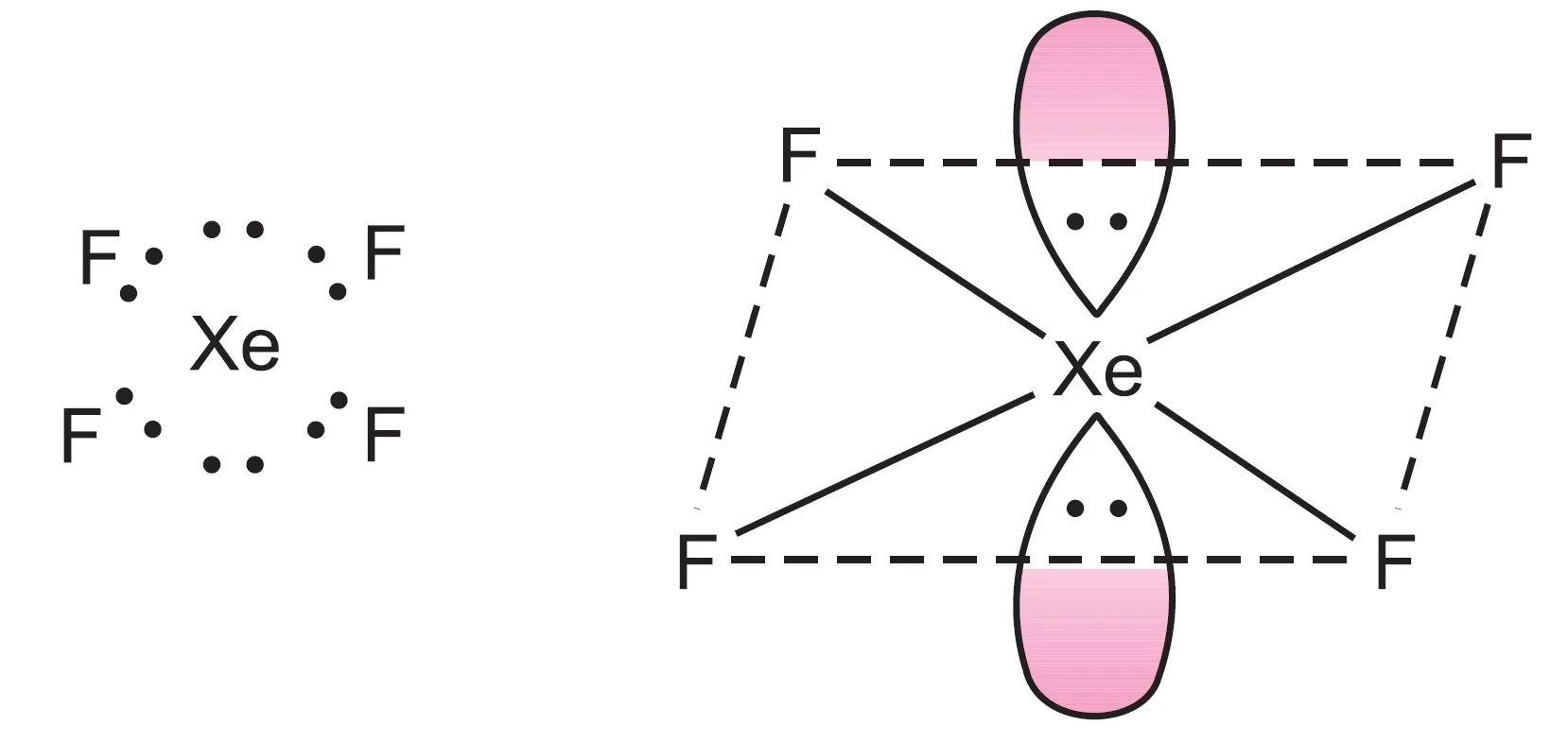
Short Answer Conceptual Type Questions (SAT)
Q1. Why does $SF_{4}$ adopt a see-saw (distorted tetrahedral) geometry?
Answer: $SF_{4}$ has 5 electron pairs (4 bp + 1 lp). The lone pair occupies an equatorial position in trigonal bipyramidal arrangement to minimize repulsion. This produces a see-saw shape with bond angles $89^{\circ}$ and $117^{\circ}$.
Q2. Why is $ClF_{3}$ T-shaped instead of trigonal planar?
Answer: $ClF_{3}$ has 5 electron pairs (3 bp + 2 lp). Both lone pairs occupy equatorial positions to reduce repulsions. This leaves 3 bond pairs forming a T-shaped geometry.
Q3. Why is $XeF_{2}$ linear even though it has 5 electron pairs?
Answer: $XeF_{2}$ has 5 electron pairs (2 bp + 3 lp). The 3 lone pairs occupy equatorial positions at $120^{\circ}$ from each other, cancelling out repulsions. Thus, the molecule is linear.
Q4. Why does $BrF_{5}$ adopt square pyramidal geometry?
Answer: $BrF_{5}$ has 6 electron pairs (5 bp + 1 lp). Octahedral arrangement is expected, but the lone pair occupies one position → square pyramidal geometry.
Q5. Why is $XeF_{4}$ square planar instead of tetrahedral?
Answer: $XeF_{4}$ has 6 electron pairs (4 bp + 2 lp). Two lone pairs occupy opposite positions in octahedral geometry, canceling repulsions → square planar geometry.
Multiple Choice Questions (MCQs) With Answers and Explanation
Q1. Which of the following has a see-saw geometry?
(a) $SF_{4}$
(b) $ClF_{3}$
(c) $XeF_{2}$
(d) $XeF_{4}$
Answer: (a) $SF_{4}$
Explanation: $SF_{4}$ has 4 bp + 1 lp → see-saw geometry.
Q2. The bond angle in $ClF_{3}$ is approximately:
(a) $120^{\circ}$
(b) $109.5^{\circ}$
(c) $87.6^{\circ}$
(d) $180^{\circ}$
Answer: (c) $87.6^{\circ}$
Explanation: Lone pairs at equatorial positions force the F atoms close, making angle slightly less than $90^{\circ}$.
Q3. Which molecule is linear?
(a) $SF_{4}$
(b) $ClF_{3}$
(c) $XeF_{2}$
(d) $BrF_{5}$
Answer: (c) $XeF_{2}$
Explanation: 2 bp + 3 lp, with lone pairs arranged equatorially → linear geometry.
Q4. Which molecule has square pyramidal geometry?
(a) $SF_{4}$
(b) $BrF_{5}$
(c) $XeF_{4}$
(d) $ClF_{3}$
Answer: (b) $BrF_{5}$
Explanation: 5 bp + 1 lp in octahedral arrangement → square pyramidal.
Q5. Which molecule has square planar geometry?
(a) $SF_{4}$
(b) $ClF_{3}$
(c) $XeF_{4}$
(d) $BrF_{5}$
Answer: (c) $XeF_{4}$
Explanation: 4 bp + 2 lp in octahedral arrangement → square planar.
Assertion–Reason Type Questions With Answers and Explanation
Q1.
Assertion (A): $SF_{4}$ has see-saw geometry.
Reason (R): Lone pair occupies equatorial position in trigonal bipyramidal arrangement.
Answer: Both A and R are true, and R is the correct explanation.
Q2.
Assertion (A): $ClF_{3}$ molecule is T-shaped.
Reason (R): Two lone pairs occupy axial positions in trigonal bipyramidal geometry.
Answer: A is true, R is false.
Explanation: Lone pairs actually occupy equatorial positions, not axial.
Q3.
Assertion (A): $XeF_{2}$ has linear geometry.
Reason (R): Three lone pairs occupy equatorial positions and cancel repulsions.
Answer: Both A and R are true, and R is the correct explanation.
Q4.
Assertion (A): $BrF_{5}$ is square pyramidal.
Reason (R): It has six bond pairs around Br.
Answer: A is true, R is false.
Explanation: $BrF_{5}$ has 5 bp + 1 lp, not 6 bp.
Q5.
Assertion (A): $XeF_{4}$ has square planar geometry.
Reason (R): Two lone pairs are opposite each other in octahedral geometry.
Answer: Both A and R are true, and R is the correct explanation.
Case Study Based Questions
Case Study:
Molecules with five or six electron pairs show distorted geometries when lone pairs are present.
- $SF_{4}$ has 4 bp + 1 lp → see-saw shape.
- $ClF_{3}$ has 3 bp + 2 lp → T-shaped.
- $XeF_{2}$ has 2 bp + 3 lp → linear.
- $BrF_{5}$ has 5 bp + 1 lp → square pyramidal.
- $XeF_{4}$ has 4 bp + 2 lp → square planar.
Questions:
- Which molecule among the above has linear geometry?
Answer: $XeF_{2}$. - Why does $ClF_{3}$ adopt a T-shape?
Answer: Because two lone pairs occupy equatorial positions, leaving 3 bond pairs → T-shaped geometry. - Which molecule has the highest number of lone pairs?
Answer: $XeF_{2}$ (3 lone pairs). - Distinguish between the geometries of $BrF_{5}$ and $XeF_{4}$.
Answer: $BrF_{5}$ → square pyramidal (5 bp + 1 lp).
$XeF_{4}$ → square planar (4 bp + 2 lp). - Arrange $SF_{4}, ClF_{3}, XeF_{2}$ in decreasing order of number of bond pairs.
Answer: $SF_{4}$ (4 bp) > $ClF_{3}$ (3 bp) > $XeF_{2}$ (2 bp).


