Anand Classes presents detailed Class 11 Chemistry notes on Dipole Moment Significance and Applications along with the concept of Polar and Non-Polar Molecules. Learn how dipole moment helps in determining the polarity of molecules, predicting molecular geometry, estimating bond character, and understanding physical properties like solubility and boiling point. These notes also cover differences between polar and non-polar molecules with examples, solved Q&A, MCQs, and practice questions for NEET, JEE, and CBSE exams. Click the print button to download study material and notes.
Importance of Dipole Moment
Q1. What is the importance of dipole moment in understanding the nature of chemical bond?
Dipole moment plays very important role in understanding the nature of chemical bond.
A few applications are given below :
Distinction between polar and non-polar molecules.
Q2. How does dipole moment help in the distinction between polar and non-polar molecules?
The measurement of dipole moment can help us to distinguish between polar and non-polar molecules.
- Non-polar molecules have zero dipole moment
- Polar molecules have some value of dipole moment
Example:
- H2 molecule with zero dipole moment is a non-polar molecule
- HCl molecule having dipole moment of 1.07 D is a polar molecule
Q3. Give examples of non-polar and polar molecules based on dipole moment values.
- Non-polar molecules (Dipole moment = 0):
H2, O2, Cl2, N2, CO2, BeF2, BF3, CH4 - Polar molecules (Dipole moment ≠ 0):
H2O ; (1.85 , D)
HF ; (1.78 D)
HCl ; (1.07 D)
H2S ; (0.95 D)
Degree of polarity in a molecule
Q4. How does dipole moment indicate the degree of polarity in a molecule?
Dipole moment measurement also gives an idea about the degree of polarity, especially in a diatomic molecule.
- The greater the dipole moment, the greater is the polarity in such a molecule.
- Example: HF (1.78 D) is more polar than HCl (1.07 D).
Shapes of molecules
Q5. How does dipole moment help in determining the shapes of molecules?
In case of molecules containing more than two atoms, the dipole moment not only depends upon the individual dipole moments of the bonds but also on the arrangement of bonds.
- Thus, dipole moment is used to find the shapes of molecules.
Examples:
- The dipole moment of water is 1.85 D while that of carbon dioxide is zero.
- This means that carbon dioxide is a linear molecule in which the individual dipole moments of the two C=O bonds cancel each other.
- On the other hand, water is not linear but has an angular shape with the resultant dipole moment of the two bond dipoles = 1.84 D.
Ionic character in a molecule
Q6. How does dipole moment help in understanding the ionic character in a molecule?
Knowing the electronegativities of atoms involved in a molecule, it is possible to predict the nature of the chemical bond formed.
- If the difference in electronegativities of two atoms is large, the bond will be highly polar.
- As an extreme case, when the electron is completely transferred from one atom to another, an ionic bond is formed.
- Therefore, the ionic bond is regarded as an extreme case of covalent bond.
- The greater the difference in electronegativities of the bonded atoms, the higher is the ionic character.
Q7. What is the role of electronegativity difference in determining ionic character?
- It has been observed that when the electronegativity difference between two atoms is 1.7, then the bond is 50% ionic and 50% covalent.
- If the difference is more than 1.7, then the chemical bond formed is predominantly ionic (more than 50% ionic character).
- If the difference is less than 1.7, the bond formed is mainly covalent.
- Larger the electronegativity difference, larger will be the ionic character.
Examples:
- Bond between Cs (0.7) and F (4.0) is more ionic than bond between Li (1.0) and F (4.0).
- Bond between C (2.5) and H (2.1) or C (2.5) and Cl (3.0) is almost covalent because the electronegativity difference is small.
Q8. How is the percentage of ionic character calculated from dipole moment?
The percentage of ionic character can be calculated from the ratio of the observed dipole moment to the dipole moment for complete electron transfer (100% ionic character).
Example (HCl molecule):
- Observed dipole moment = 1.07 D
- Bond length = 1.275 Å
Assuming 100% ionic character, the charge developed on H and Cl atoms would be: q = 4.8 x 10-10 esu
Therefore, dipole moment for 100% ionic character will be:
µ (ionic) = q x d = (4.8 x 10-10 esu) x (1.275 x 10-8 cm)
µ (ionic) = 6.12 x 10-18 esu cm = 6.12 D
Hence, %( ionic character)
$$ = \frac{\text{Observed dipole moment}}{\text{Dipole moment for complete ionic character}} \times 100$$
$$= \frac{1.07}{6.12} \times 100 = 17.48%$$
Distinguish between cis- and transisomers
Q9. How can dipole moment be used to distinguish between cis- and trans- isomers?
Dipole moment measurements help to distinguish between cis- and trans- isomers because:
- Cis-isomer usually has a higher dipole moment than the trans-isomer.
- In trans-isomer of 1,2-dichloroethene, the dipole moment is less because the bond moments of C–Cl bonds cancel each other.
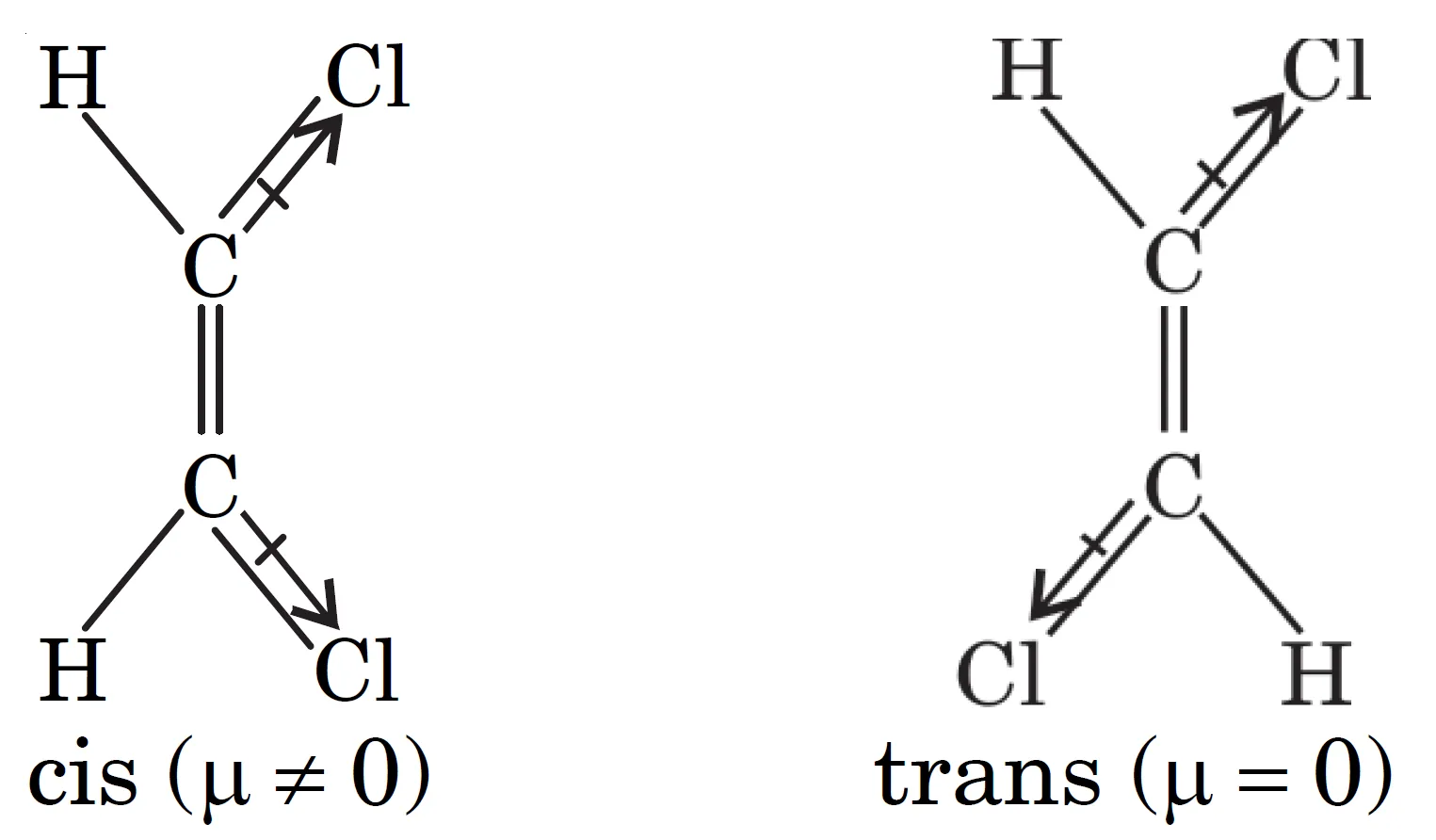
Q10. Why is the dipole moment of trans-isomer of 1,2-dichloroethene less than that of cis-isomer?
- In the cis-isomer, the two C–Cl bond dipoles are oriented in the same direction.
- Hence, they add up, giving a higher net dipole moment.
- In the trans-isomer, the two C–Cl bond dipoles are oriented in opposite directions.
- As a result, they partially (or completely) cancel each other.
- Therefore, the net dipole moment is less than that of the cis-isomer.
Order of dipole moment:
$$\mu_{trans} < \mu_{cis}$$
Distinguish between ortho, meta and para isomers
Q11. How can dipole moment be used to distinguish between ortho, meta and para isomers?
Dipole moment measurements help to distinguish between o-, m- and p- isomers because:
- The dipole moment of p-isomer is zero
- The dipole moment of o-isomer is more than that of m-isomer
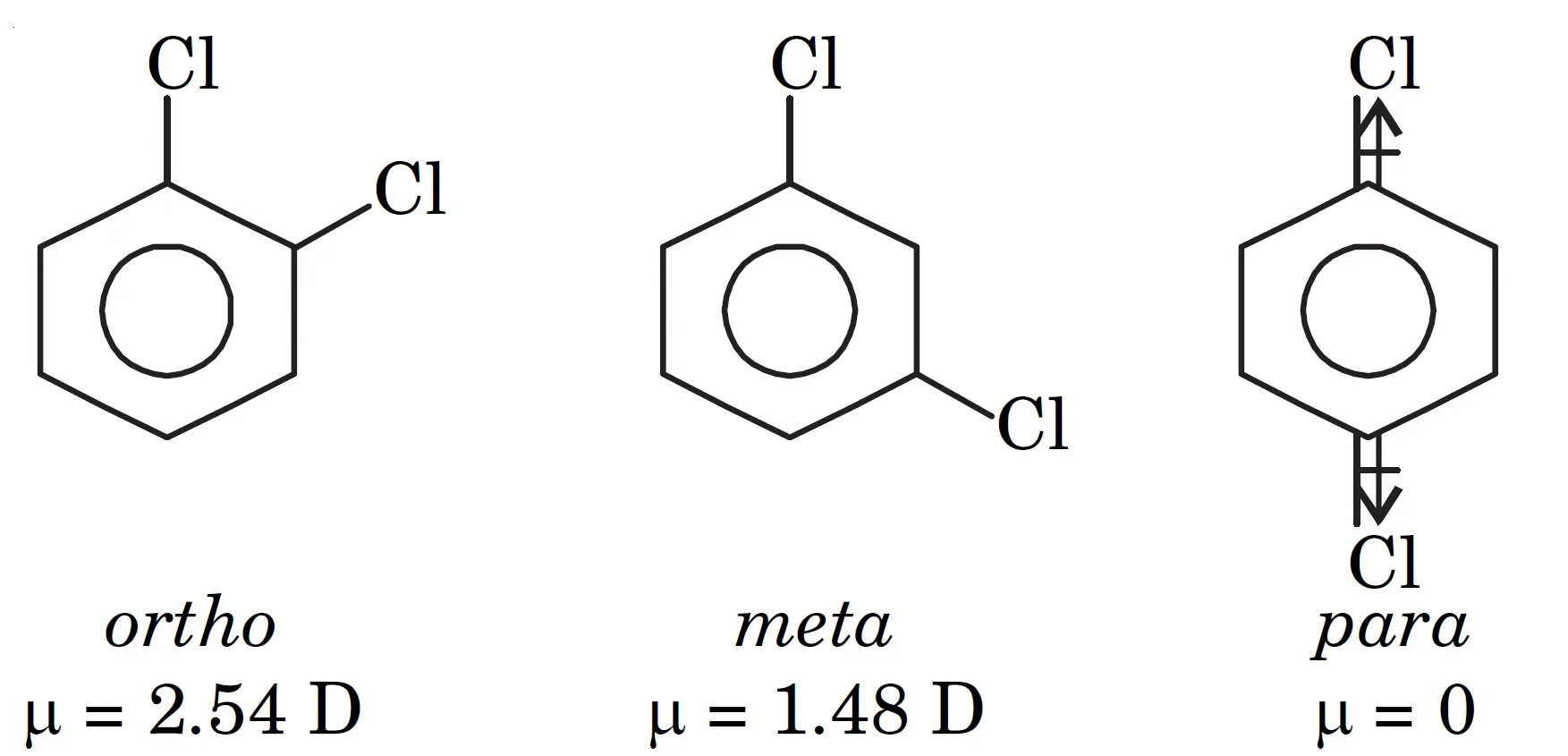
Example:
For dichlorobenzene :
- o-dichlorobenzene → higher dipole moment
- m-dichlorobenzene → moderate dipole moment
- p-dichlorobenzene → zero dipole moment
Q12. Why is the dipole moment of p-dichlorobenzene zero and that of o-dichlorobenzene more than that of m-dichlorobenzene?
- In p-isomer (para-isomer) of dichlorobenzene, the two substituents are opposite to each other on the benzene ring. (see in above diagram)
- The bond dipoles are equal in magnitude but opposite in direction, hence they cancel each other.
- Therefore, the net dipole moment = 0.
- In o-isomer (ortho-isomer) dichlorobenzene, the two substituents are adjacent, so their bond dipoles do not cancel completely.
- Instead, they combine to give a higher resultant dipole moment.
- In m-isomer (meta-isomer) dichlorobenzene, the bond dipoles are at 120°, so partial cancellation occurs.
- The resultant dipole moment is less than that of o-isomer but greater than zero.
Order of dipole moment: µp = 0 < µm < µo
📝 Multiple Choice Questions (MCQs) on Dipole Moment Significance (Applications)
MCQ1. Which of the following molecules has zero dipole moment?
(a) HCl
(b) H2O
(c) CO2
(d) NH3
Answer: (c) CO2
Explanation: In CO2, the two C=O bond dipoles cancel each other due to the linear arrangement, hence µ = 0.
MCQ2. The dipole moment of HF is 1.78 D while that of HCl is 1.07 D. This indicates that:
(a) HF is more polar than HCl
(b) HF is less polar than HCl
(c) Both have equal polarity
(d) Both are non-polar
Answer: (a) HF is more polar than HCl
MCQ3. In dichlorobenzene, which isomer has zero dipole moment?
(a) Ortho
(b) Meta
(c) Para
(d) All have non-zero dipole moments
Answer: (c) Para
MCQ4. Which of the following bonds has the highest ionic character?
(a) C–H
(b) C–Cl
(c) Li–F
(d) Cs–F
Answer: (d) Cs–F
Explanation: Cs (0.7) and F (4.0) have the largest electronegativity difference, hence maximum ionic character.
MCQ5. In 1,2-dichloroethene, the dipole moment is:
(a) Higher in trans-isomer than cis-isomer
(b) Higher in cis-isomer than trans-isomer
(c) Equal in cis- and trans-isomer
(d) Zero in both cases
Answer: (b) Higher in cis-isomer than trans-isomer
📝 Assertion–Reason Questions based on Dipole Moment Significance (Applications)
AR1.
Assertion (A): The dipole moment of CO2 is zero.
Reason (R): CO2 is a linear molecule and the bond dipoles of the two C=O bonds cancel each other.
Options:
(a) Both A and R are true, and R is the correct explanation of A
(b) Both A and R are true, but R is not the correct explanation of A
(c) A is true, R is false
(d) A is false, R is true
Answer: (a)
AR2.
Assertion (A): The dipole moment of p-dichlorobenzene is zero.
Reason (R): The two C–Cl bond dipoles are equal and opposite, hence cancel each other.
Answer: (a) Both A and R are true, and R is the correct explanation of A.
AR3.
Assertion (A): The cis-isomer of 1,2-dichloroethene has higher dipole moment than trans-isomer.
Reason (R): In cis-isomer, the C–Cl bond dipoles add up, whereas in trans-isomer they oppose each other.
Answer: (a)
AR4.
Assertion (A): If the electronegativity difference between two bonded atoms is 1.7, then the bond has about 50% ionic character.
Reason (R): The greater the electronegativity difference, the greater is the ionic character of the bond.
Answer: (a)
📝 Case Study Question based on Dipole Moment Significance (Applications)
Read the passage and answer the following questions:
Dipole moment is an important property that helps in determining the polarity, shape, ionic character, and isomeric differences in molecules. For example, CO2 has zero dipole moment due to cancellation of bond dipoles in its linear shape, whereas H2O has a dipole moment of 1.85 D because of its bent shape. Dipole moment can also be used to distinguish between cis- and trans-isomers (cis-isomer has higher dipole moment than trans) and o-, m-, p-isomers of dichlorobenzene (para has zero dipole moment, ortho has highest). Additionally, the ionic character of a molecule can be estimated by comparing its observed dipole moment with the theoretical dipole moment for complete ionic separation.
Questions:
(i) Why does H₂O have a dipole moment of 1.85 D while CO2 has zero?
Answer: H2O is bent, so bond dipoles add up; CO2 is linear, so bond dipoles cancel.
(ii) Which has greater dipole moment: cis- or trans-isomer of 1,2-dichloroethene? Why?
Answer: Cis-isomer; because dipoles add up, while in trans they cancel.
(iii) Arrange the following in increasing dipole moment: o-dichlorobenzene, m-dichlorobenzene, p-dichlorobenzene.
Answer: Order of dipole moment: µp = 0 < µm < µo
(iv) Calculate the % ionic character of HCl if observed dipole moment is 1.07 D and ionic dipole moment is 6.12 D.
Answer:
%ionic character = (1.07/6.12) x 100 = 17.48%