Anand Classes presents detailed Class 11 Chemistry notes on Bond Angle, Bond Enthalpy, and Bond Order with clear explanations, solved Q&A, and exam-focused MCQs. Learn how bond angle determines molecular shape, how bond enthalpy measures the strength of a chemical bond, and how bond order relates to stability and bond length. These well-organized notes are ideal for CBSE, NEET, and JEE aspirants looking for quick revision and concept clarity. Click the print button to download study material and notes.
What is Bond Angle?
Bond angle may be defined as the average angle between the orbitals containing bonding electron pairs around the central atom in a molecule.
It is expressed in degree/minute/second.
Bond angle gives an idea about the distribution of orbitals around the central atom in a molecule and therefore determines the shape of a molecule.
Examples:
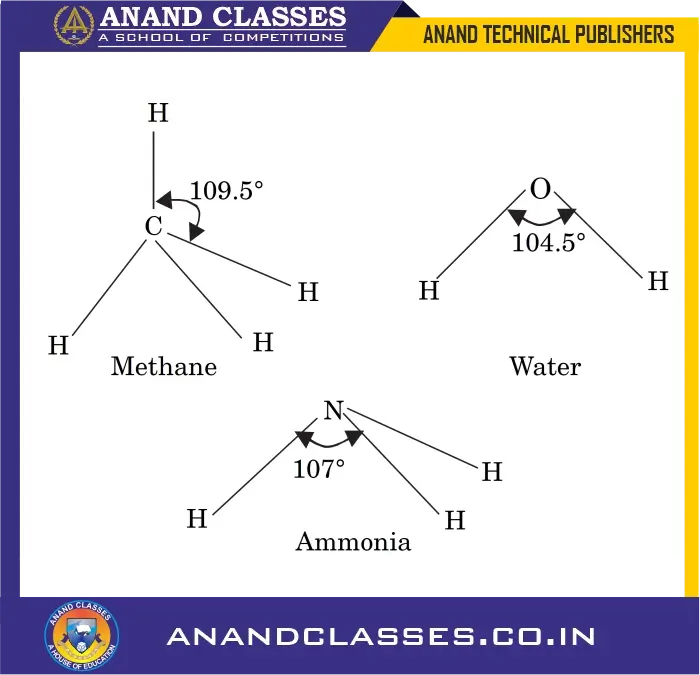
- In methane, the $H—C—H$ bond angle is 109.5°.
- In water, the $H—O—H$ bond angle is 104.5°.
- In ammonia, the $H—N—H$ bond angle is 107°.
What is Bond Enthalpy (Bond Dissociation Enthalpy)?
When a bond is formed between atoms, energy is released, meaning bonded atoms have lower energy than separated atoms.
Obviously, the same amount of energy is required to break the bond, called bond dissociation enthalpy (a measure of bond strength).
Definition:
Bond dissociation enthalpy is the amount of energy required to break one mole of bonds of a particular type between atoms in the gaseous state.
It is expressed in kJ mol$^{-1}$.
Examples:
$$
H_2(g) ; \rightarrow ; H(g) + H(g), \quad \Delta_a H^\circ = 435.8 ,\text{kJ mol}^{-1}
$$
$$
Cl_2(g) ; \rightarrow ; Cl(g) + Cl(g), \quad \Delta_a H^\circ = 243.5 ,\text{kJ mol}^{-1}
$$
$$
I_2(g) ; \rightarrow ; I(g) + I(g), \quad \Delta_a H^\circ = 151.0 ,\text{kJ mol}^{-1}
$$
$$
HI(g) ; \rightarrow ; H(g) + I(g), \quad \Delta_a H^\circ = 298.3 ,\text{kJ mol}^{-1}
$$
For double and triple bonds:
$$
O_2(g) ; \rightarrow ; O(g) + O(g), \quad \Delta_a H^\circ = 498.0 ,\text{kJ mol}^{-1}
$$
$$
N_2(g) ; \rightarrow ; N(g) + N(g), \quad \Delta_a H^\circ = 946.0 ,\text{kJ mol}^{-1}
$$
It may be noted that larger the bond dissociation enthalpy, stronger will be the bond in the molecule.
Larger bond dissociation enthalpy → stronger bond.
What are the Bond Dissociation Enthalpies of Some Common Bonds?
| Bond | Bond dissociation enthalpy (kJ mol$^{-1}$) |
|---|---|
| H—H | 435.8 |
| H—Cl | 431.7 |
| H—Br | 366.1 |
| H—I | 298.3 |
| F—F | 158.1 |
| Cl—Cl | 243.5 |
| Br—Br | 192.8 |
| I—I | 151.0 |
| N—H | 389.2 |
| O—H | 464 |
| O=O | 498 |
| C—H | 414 |
| C—C | 433 |
| C=C | 619 |
| C≡C | 836 |
| N≡N | 946.0 |
What Factors Affect Bond Dissociation Enthalpy?
(i) How Does Atomic Size Affect Bond Enthalpy?
The smaller the size of the bonded atoms, the stronger is the bond. consequently, larger is the value of bond dissociation enthalpy.
Smaller bonded atoms → stronger bonds → larger bond enthalpy.
Example:
- $H—H$: 435.8 kJ mol$^{-1}$
- $Cl—Cl$: 243.5 kJ mol$^{-1}$
So, $H—H$ is stronger because H is smaller than Cl.
(ii) How Does Bond Length Affect Bond Enthalpy?
Shorter the bond length, larger is the value of bond enthalpy.
Shorter bonds → larger bond enthalpy.
Example:
- $C—C$ bond length = 154 pm, enthalpy = 433 kJ mol$^{-1}$
- $C=C$ bond length = 134 pm, enthalpy = 619 kJ mol$^{-1}$
What is Average Bond Enthalpy?
In polyatomic molecules (with more than one bond of the same type), bond enthalpies differ due to chemical environment.
So, we take the average of all bond dissociation enthalpies.
The average of the bond dissociation enthalpies is called average bond enthalpy or simply as bond enthalpy.
Example (Water): For example, in the case of water molecule, the enthalpy needed to break the two O–H bonds is not the same.
$$
H_2O(g) \rightarrow H(g) + OH(g), \quad \Delta_a H_1^\circ = 502 ,\text{kJ mol}^{-1}
$$
$$
OH(g) \rightarrow H(g) + O(g), \quad \Delta_a H_2^\circ = 427 ,\text{kJ mol}^{-1}
$$
The difference in the $\Delta_a H^\circ $ values suggests that the second O–H bond undergoes some change because of the changed chemical environment. This is the reason for some difference in energy of same O–H bond in different molecules such as CH3OH (methanol), C2H5OH (ethanol), water, etc.
Therefore for polyatomic molecules, mean or average bond enthalpy is used. It is obtained as the average of the different bond dissociation enthalpies in a molecule.
For example, for water,
Average bond enthalpy of O—H in water:
$$
\frac{502 + 427}{2} = 464.5 ,\text{kJ mol}^{-1}
$$
Thus, bond enthalpy = average enthalpy required to break bonds of a given type in one mole of gaseous molecules.
For diatomic molecules, bond enthalpy = bond dissociation enthalpy.
What is Bond Order?
Definition:
The bond order is defined as the number of bonds between two atoms in a molecule.
Bond order = number of bonds between two atoms in a molecule.
Examples:
- $H_2$: bond order = 1
- $O_2$: bond order = 2
- $N_2$: bond order = 3
- $CO$: bond order = 3
It may be noted that isoelectronic species (molecules and ions) have same bond order :
- $F_2$, $O_2^{2-}$ → bond order = 1
- $N_2$, $CO$, $NO^+$ → bond order = 3
It may be remembered that in general, With increase in bond order, bond enthalpy increases and bond length decreases.
General Rule:
- With ↑ bond order → bond enthalpy ↑, bond length ↓
Short Answer Conceptual Type Questions (SAT)
Q1. What is bond angle?
Answer:
Bond angle is the average angle between the orbitals containing bonding electron pairs around the central atom in a molecule. It is expressed in degrees and determines the shape of the molecule.
Q2. Give the bond angles of methane, water, and ammonia.
Answer:
- Methane ($CH_4$): $H—C—H = 109.5^\circ$
- Water ($H_2O$): $H—O—H = 104.5^\circ$
- Ammonia ($NH_3$): $H—N—H = 107^\circ$
Q3. Define bond dissociation enthalpy.
Answer:
Bond dissociation enthalpy is the amount of energy required to break one mole of bonds of a particular type in the gaseous state.
Q4. How does bond length affect bond enthalpy?
Answer:
Bond enthalpy is inversely proportional to bond length. Shorter bonds are stronger and have higher bond enthalpy.
Q5. Define bond order. Give examples.
Answer:
Bond order is the number of chemical bonds between two atoms in a molecule.
- $H_2$: Bond order = 1
- $O_2$: Bond order = 2
- $N_2$: Bond order = 3
Multiple Choice Questions (MCQs) With Answers and Explanation
Q1. The $H—O—H$ bond angle in water is:
(a) $104.5^\circ$
(b) $107^\circ$
(c) $109.5^\circ$
(d) $120^\circ$
Answer: Correct option (a)
Explanation: Water has a bent shape due to lone pair–bond pair repulsion. Thus, bond angle = $104.5^\circ$.
Q2. Which bond has the highest bond dissociation enthalpy?
(a) $C—C$
(b) $C=C$
(c) $C≡C$
(d) $C—H$
Answer: Correct option (c)
Explanation:
- $C—C = 433$ kJ mol$^{-1}$
- $C=C = 619$ kJ mol$^{-1}$
- $C≡C = 836$ kJ mol$^{-1}$ (highest)
Q3. Which of the following species has bond order = 3?
(a) $F_2$
(b) $O_2^{2-}$
(c) $N_2$
(d) $O_2$
Answer: Correct option (c)
Explanation: $N_2$, $CO$, and $NO^+$ are isoelectronic species with bond order 3.
Assertion-Reason Type Questions With Answers and Explanation
Q1.
Assertion (A): The bond angle in $CH_4$ is greater than that in $H_2O$.
Reason (R): Lone pairs on oxygen in $H_2O$ repel more strongly than bond pairs, reducing the bond angle.
Answer: Both A and R are true, and R is the correct explanation of A.
Q2.
Assertion (A): The bond enthalpy of $C≡C$ is greater than that of $C=C$.
Reason (R): Multiple bonds are shorter and stronger than single bonds.
Answer: Both A and R are true, and R is the correct explanation of A.
Q3.
Assertion (A): $O_2^{2-}$ has lower bond enthalpy than $O_2$.
Reason (R): Addition of electrons decreases bond order.
Answer: Both A and R are true, and R is the correct explanation of A.
Case Study Based Question
Passage:
The bond angles and bond enthalpies of molecules provide important insights into molecular structure and stability. In methane ($CH_4$), the bond angle is $109.5^circ$, while in ammonia ($NH_3$) it is $107^circ$ and in water ($H_2O$) it is $104.5^circ$. Bond dissociation enthalpy values show that triple bonds are stronger than double bonds, and double bonds are stronger than single bonds. For example:
- $C—C$: 433 kJ mol$^{-1}$
- $C=C$: 619 kJ mol$^{-1}$
- $C≡C$: 836 kJ mol$^{-1}$
Bond order explains these variations, as higher bond order corresponds to stronger and shorter bonds.
Questions:
- Arrange $CH_4$, $NH_3$, and $H_2O$ in decreasing order of bond angle.
- Compare bond enthalpy of $C—C$, $C=C$, and $C≡C$.
- Why is bond enthalpy of $O_2^{2-}$ less than that of $O_2$?
Answers:
- $CH_4 (109.5^\circ) > NH_3 (107^\circ) > H_2O (104.5^\circ)$
- $C≡C (836) > C=C (619) > C—C (433)$
- $O_2^{2-}$ has bond order = 1, while $O_2$ has bond order = 2. Lower bond order → weaker bond → lower bond enthalpy.


