Anand Classes brings you detailed Class 11 Chemistry notes on Co-ordinate (Dative) Covalent Bond with clear explanations, examples, and important MCQs to help you master this fundamental concept. Learn how a single atom donates an electron pair to form a stable covalent bond, understand real-life examples, and practice multiple-choice questions designed for NEET, JEE, and CBSE exams. These well-structured notes make complex topics easy to grasp and are perfect for quick revision and exam preparation. Click the print button to download study material and notes.
What is a Co-ordinate Covalent Bond?
So far, we have considered that in single covalent bonds each atom contributes one electron. In some cases, the shared pair of electrons is provided entirely by one atom, while the other atom merely participates in sharing.
In 1921, Perkin suggested another covalent type bond in which both the electrons in the shared pair come from one atom. This is called a coordinate covalent bond.
A Co-ordinate bond is a type of alternate covalent bond that is formed by sharing of an electron pair from a single atom. Both shared electrons are donated by the same atom. It is also called a dative bond.
- Co-ordinate covalent bonds are usually strong bonds. This is because the bonds are identical to any other interatomic bonds.
- The coordinate bond is a directional bond.
- Co-ordinate covalent bonds are usually formed in reactions that involve two non-metals, such as a hydrogen atom or during bond formation between metal ions and ligands (Electron donar atoms or ions).
How is a Co-ordinate Covalent Bond Formed?
Co-ordinate Covalent Bond is formed between atoms (or ions), one of which is deficient in at least two electrons, while the other atom has already acquired a stable noble gas configuration (octet).
- The atom which contributes the electrons is called the donor.
- The atom which only shares the electron pair is called the acceptor.
This bond is usually represented by an arrow ($\longrightarrow$) pointing from donor to acceptor atom.
This type of bond is also known as:
- Dative bond
- Donor-acceptor bond
- Semi-polar bond
- Co-ionic bond
Note : It may be remembered that in the modern terminology, there is no distinction between a covalent bond and a coordinate bond. Once the coordinate bond is formed, it cannot be distinguished from the normal covalent bond.
Refer this diagram for following examples.
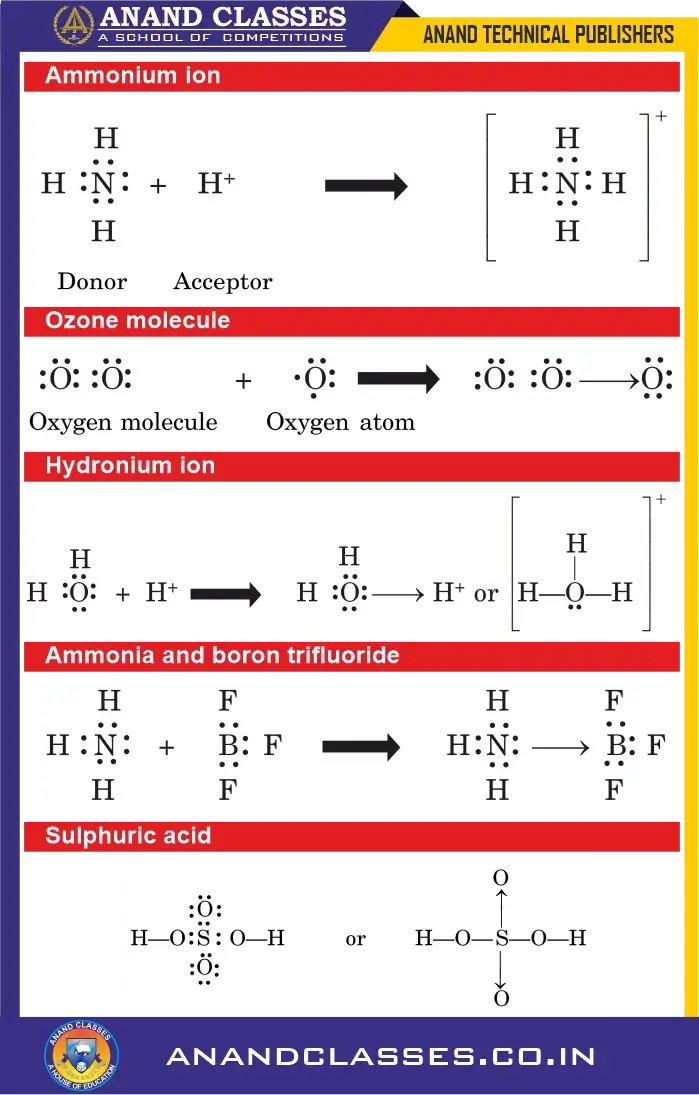
Example: Formation of Ammonium Ion (NH4+)
A hydrogen ion (H+) combines with an ammonia molecule (NH3) by a coordinate covalent bond to form the ammonium ion (NH4+) :
$$
\text{NH}_3 + \text{H}^+ \longrightarrow \text{NH}_4^+
$$
(Refer above diagram)
In modern terminology, there is no distinction between a covalent bond and a coordinate bond. Once formed, a coordinate bond cannot be distinguished from a normal covalent bond.
What are Examples of Co-ordinate Covalent Bonds?
(i) Ozone Molecule (O3)
A molecule of oxygen contains two oxygen atoms joined by double covalent bond so that octet of each of the two atoms is complete. Now, if an atom of oxygen having six electrons comes close to oxygen molecule, the new oxygen atom may share a lone pair of electrons of one of the oxygen atoms of the oxygen molecule. This results in the formation of a coordinate bond as shown below :
$$
\text{O} = \text{O} : ; + ; \text{O} \longrightarrow \text{O}_3
$$
(Refer above diagram)
(ii) Hydronium Ion (H3O+)
Water ($text{H}_2text{O}$) has two lone pairs of electrons on oxygen, while $text{H}^+$ has an empty $1s$ orbital. Oxygen donates a lone pair to $\text{H}^+$, forming a coordinate bond:
$$
\text{H}_2\text{O} + \text{H}^+ \longrightarrow \text{H}_3\text{O}^+
$$
(Refer above diagram)
(iii) Ammonia and Boron Trifluoride ($\text{NH}_3 + \text{BF}_3$)
Ammonia has a lone pair of electrons on nitrogen, while boron trifluoride is electron-deficient. They combine through a coordinate bond:
$$
\text{NH}_3 + \text{BF}_3 \longrightarrow \text{H}_3\text{N} \longrightarrow \text{BF}_3
$$
(Refer above diagram)
(iv) Sulphuric Acid ($\text{H}_2\text{SO}_4$)
In sulphuric acid, two coordinate bonds are present.
- The bonds between sulphur and the oxygen atoms of the two –OH groups are normal single bonds.
- The bonds between sulphur and the other two oxygen atoms are coordinate bonds:
$$
\text{H}_2\text{SO}_4 : ; \text{S} \longrightarrow \text{O}
$$
(Refer above diagram)
Short Answer Conceptual Type Questions (SAT) on co-ordinate covalent bond
Q1. Define a co-ordinate covalent bond.
Answer:
A co-ordinate covalent bond is a type of covalent bond in which both electrons in the shared pair are donated by the same atom (donor), while the other atom (acceptor) only participates in sharing.
Q2. Give two examples of compounds containing co-ordinate covalent bonds.
Answer:
Examples:
- Ammonium ion ($\text{NH}_4^+$)
- Hydronium ion ($\text{H}_3\text{O}^+$)
Q3. What are the characteristics of Coordinate Covalent Bond
Answer:
- In this type of bonding, the atom that shares an electron pair from itself is termed as the donor.
- The other atom which accepts these shared pair of electrons is known as a receptor or acceptor.
- The bond is represented with an arrow →, pointing towards the acceptor from the donor atom.
- After sharing of electron pairs, each atom gets stability.
- This type of bonding is central to the Lewis theory.
- Getting a good understanding of co-ordinate covalent bonds can help in properly designing complex organic molecules.
Q4. Is co-ordinate bonding a strong bond?
Answer:
Co-ordinate covalent bonds are usually strong bonds. This is because the bonds are identical to any other interatomic bonds.
Q5. Is the coordinate bond directional?
Answer:
Yes, the coordinate bond is a directional bond.
Multiple Choice Questions (MCQs) With Answers and Explanation on co-ordinate covalent bond
Q1. Which of the following contains a co-ordinate covalent bond?
(a) $\text{H}_2$
(b) $\text{O}_2$
(c) $\text{NH}_4^+$
(d) $\text{Cl}_2$
Answer: Correct option (c)
Explanation:
In $\text{NH}_4^+$, a hydrogen ion ($\text{H}^+$) accepts a lone pair of electrons from $\text{NH}_3$ to form a co-ordinate covalent bond.
Q2. A co-ordinate covalent bond is also called:
(a) Ionic bond
(b) Donor-acceptor bond
(c) Metallic bond
(d) Hydrogen bond
Answer: Correct option (b)
Explanation:
Since one atom donates both electrons and the other accepts them, the co-ordinate bond is also called a donor-acceptor bond.
Assertion Reason Type Questions With Answers and Explanation on co-ordinate covalent bond
Q1.
Assertion (A): In $\text{NH}_4^+$ ion, the bond between nitrogen and the fourth hydrogen is a co-ordinate covalent bond.
Reason (R): Both the bonding electrons are contributed by the nitrogen atom.
Answer: Both Assertion (A) and Reason (R) are true, and Reason (R) is the correct explanation of Assertion (A).
Explanation:
Nitrogen in $\text{NH}_3$ donates its lone pair of electrons to $\text{H}^+$, forming a co-ordinate covalent bond.
Case Study Based Question on co-ordinate covalent bond
Read the following passage and answer the questions:
Ozone ($\text{O}_3$) is formed when an oxygen molecule reacts with an oxygen atom. One oxygen atom shares a lone pair of electrons with the oxygen molecule through a co-ordinate covalent bond. Once formed, this bond is indistinguishable from a normal covalent bond.
Questions:
- What type of bond is formed in ozone apart from the normal covalent bond?
- How is a co-ordinate covalent bond represented?
- Write the equation for the formation of ozone.
Answers:
- A co-ordinate covalent bond.
- It is represented by an arrow ($\longrightarrow$) from donor to acceptor atom.
- $$
\text{O}_2 + \text{O} \longrightarrow \text{O}_3
$$


