Anand Classes provides detailed notes on Exceptions to the Octet Rule and its Limitations for Class 11 Chemistry. While the octet rule explains stability of covalent compounds, several exceptions exist such as molecules with incomplete octet ($BF_3$, $BeCl_2$), expanded octet ($PCl_5$, $SF_6$), odd-electron molecules ($NO$, $NO_2$), and cases where the rule fails to explain stability (resonance, metallic bonding). These notes cover theory, solved examples, MCQs, assertion–reason, and case studies useful for CBSE, JEE, and NEET exam preparation. Click the print button to download study material and notes.
What are the Exceptions to the Octet Rule and its Drawbacks ?
The octet rule states that atoms tend to gain, lose, or share electrons to achieve a full valence shell of 8 electrons. While this rule explains the stability of many compounds, there are important exceptions.
1. Hydrogen Molecule (H2)
Hydrogen has one electron in its first shell (n=1). The first shell can accommodate only 2 electrons, so hydrogen needs only one more electron to achieve stability:
$$
H(1s^1) + H(1s^1) \longrightarrow H_2 \ (1s^2)
$$
- Explanation: Hydrogen achieves a duplet rather than an octet.
2. Incomplete Octet of the Central Atom
The octet rule cannot explain the formation of certain molecules of lithium, beryllium, boron, aluminium, etc. (LiCl, BeH2, BeCl2, BH3, BF3) in which the central atom has less than eight electrons in the valence shell. These are electron deficient compounds as shown below :
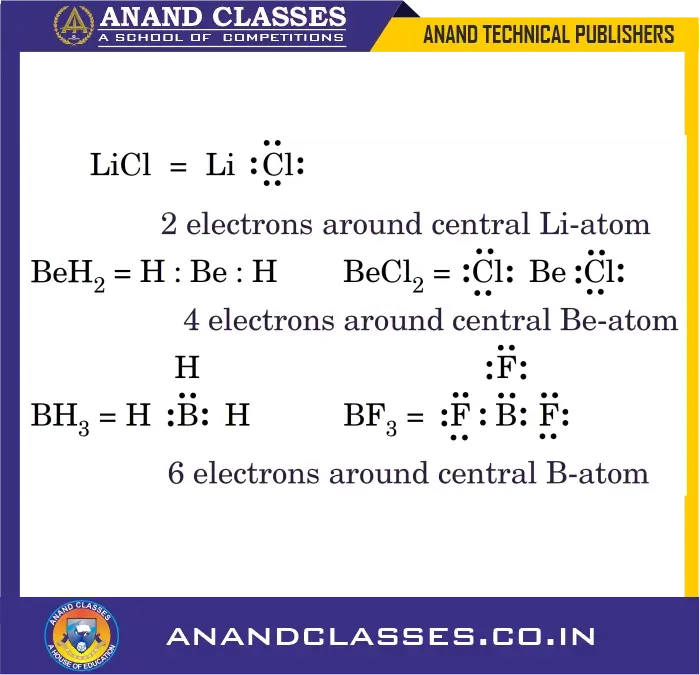
3. Expanded Octet of the Central Atom
There are many stable molecules which have more than eight electrons in their valence shells. Elements in the third period and beyond can accommodate more than 8 electrons due to the availability of d-orbitals.
For example, PF5 has ten; SF6 has twelve and IF7 has fourteen electrons around the central atoms, P, S and I respectively, as shown below :
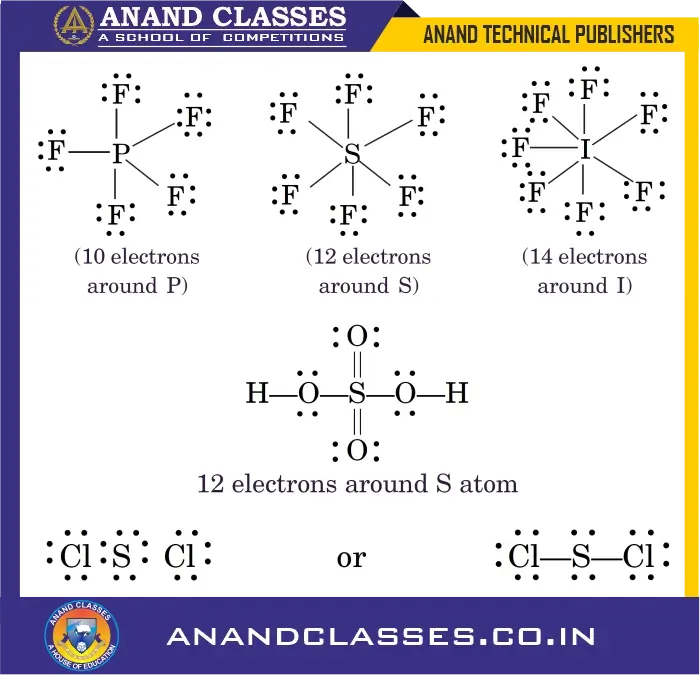
Sulphuric acid ($\mathrm{H_2SO_4}$) also shows expansion:
$$
\mathrm{O{=}S(OH)_2{=}O}
$$
Sulfur has 12 electrons around it.
Such compounds are called hypervalent compounds.
Note: Sulfur also forms octet-obeying compounds like sulfur dichloride:
$$
\mathrm{Cl-S-Cl}
$$
4. Odd-Electron Molecules
Some molecules contain an odd number of total valence electrons, preventing all atoms form completing an octet, like nitric oxide, NO and nitrogen dioxide, NO2. In these cases, octet rule is not satisfied for all the atoms.
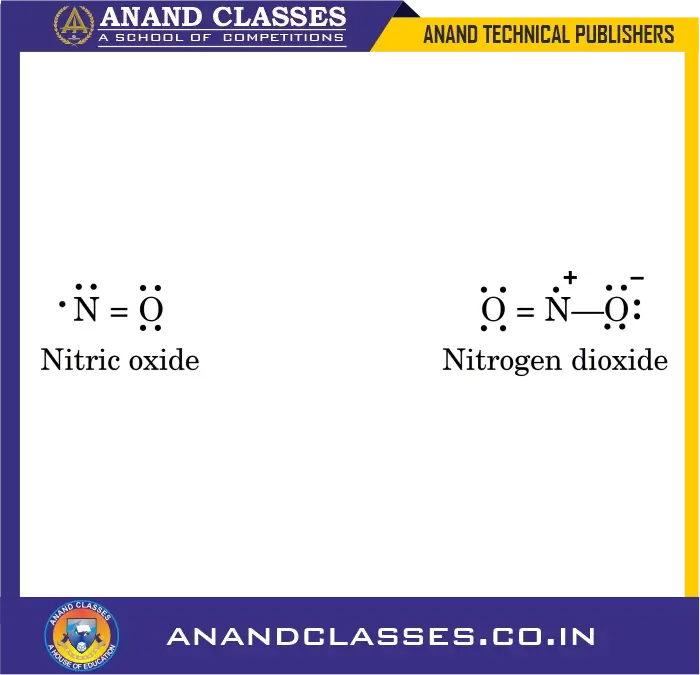
Examples:
- Nitric oxide (NO): 11 valence electrons
- Nitrogen dioxide (NO2): 17 valence electrons
The other examples of molecules having odd number of valence electrons are ozone (O3), superoxide ion (O2–) etc.
5. Noble Gas Compounds
Despite being inert, heavier noble gases form compounds due to available d-orbitals. It has been found that some noble gases (especially xenon and krypton) also combine with oxygen and fluorine to form a large number of compounds such as XeF2, KrF2, XeOF2, XeOF4, XeF6, etc.
6. Octet Rule Does Not Predict Molecular Shape
The octet rule explains electron count, but it does not determine 3D geometry or bond angles.
Molecular shapes require VSEPR theory.
7. Octet Rule Does Not Explain Stability in Terms of Energy
Some molecules satisfying the octet rule are less stable than those violating it.
- Example: $ \mathrm{BF_3} $ has an incomplete octet yet is stable due to strong B–F bonding.
Summary Table of Exceptions
| Type of Exception | Example(s) | Explanation |
|---|---|---|
| Hydrogen (duplet) | $ \mathrm{H_2} $ | Only 2 electrons required |
| Incomplete octet | $ \mathrm{LiCl,\ BeH_2,\ BH_3} $ | Central atom has <8 electrons |
| Expanded octet | $ \mathrm{PF_5,\ SF_6,\ IF_7} $ | Central atom has >8 electrons (uses d-orbitals) |
| Odd-electron molecules | $ \mathrm{NO,\ NO_2,\ O_3} $ | Molecule has odd number of electrons |
| Noble gas compounds | $ \mathrm{XeF_2,\ KrF_2} $ | Heavy noble gases expand octet |
| Energy/shape exceptions | $ \mathrm{BF_3} $ | Stability not solely based on octet rule |
Short Answer Conceptual Types Questions (SAT) on Exceptions to the Octet Rule
Q1. What is meant by an “exception to the octet rule”?
An exception to the octet rule occurs when atoms in a molecule do not follow the typical requirement of having eight electrons in their valence shell.
Examples include incomplete octets (like Be in BeH₂), expanded octets (like S in SF₆), and odd-electron molecules (like NO).
Q2. Why can elements like S, P, and Cl form expanded octets?
Elements in the third period and beyond have vacant d-orbitals that can accommodate more than 8 electrons, allowing the formation of compounds such as PF5, SF6, IF7
Q3. Why is hydrogen stable with only two electrons?
Hydrogen has only the 1s orbital (first shell), which can hold a maximum of 2 electrons.
Thus, it follows a duplet rule instead of the octet rule.
Q4. Can noble gases form compounds?
Yes. Heavy noble gases such as xenon and krypton can form compounds like XeF2, KrF2, XeOF2, XeOF4, XeF6, etc. due to their ability to expand their octet.
Multiple Choice Questions (MCQs) With Answers and Explanation on Exceptions to the Octet Rule
- Which of the following molecules contains an odd number of electrons?
(a) CO₂
(b) NO
(c) CH₄
(d) SF₆
Answer: (b) NO - Which of the following molecules has a central atom with an incomplete octet?
(a) BF₃
(b) PF₅
(c) SF₆
(d) XeF₂
Answer: (a) BF₃ - In which of the following compounds does the central atom have 12 valence electrons?
(a) SF₆
(b) PF₅
(c) IF₇
(d) NO₂
Answer: (a) SF₆
Assertion–Reason Questions With Answers and Explanation on Exceptions to the Octet Rule
- Assertion (A): SF₆ contains 12 electrons around the central sulfur atom.
Reason (R): Sulfur can expand its octet because it has vacant d-orbitals. (a) Both A and R are true, and R is the correct explanation of A.
(b) Both A and R are true, but R is not the correct explanation of A.
(c) A is true, but R is false.
(d) A is false, but R is true. Answer: (a) - Assertion (A): Hydrogen requires eight electrons to achieve stability.
Reason (R): Hydrogen belongs to group 1 and behaves like alkali metals. Answer: (d) (A is false, R is true)
Case Study based on Exceptions to the Octet Rule
Read the following passage and answer the questions:
Boron trifluoride (BF₃) is a colorless gas used as a catalyst. The boron atom forms three covalent bonds with fluorine atoms. Boron has only six electrons in its valence shell and does not complete an octet.
Questions:
- Why is BF₃ stable despite an incomplete octet?
Answer: Boron forms strong B–F covalent bonds and can accept electron pairs from donor molecules (like NH₃) to form adducts. - Name another compound where the central atom has an incomplete octet.
Answer: Beryllium chloride (BeCl₂). - Write the Lewis structure of BF₃.
Answer:
$$
\mathrm{F !-! B !-! F} \
! \mid \
F
$$
Key Takeaway
The octet rule is a helpful guideline, but molecules such as BF₃, NO, SF₆, XeF₂ and others prove that chemical stability depends on more than just completing an octet—it also involves factors like available orbitals, bond energy, and electron delocalization.


