Anand Classes provides detailed notes on Formal Charge in Chemistry for Class 11 students. Formal charge is an important concept used to identify the most stable Lewis structure of a molecule or ion. It is calculated using the formula based on valence electrons, non-bonding electrons, and bonding electrons. These notes include the definition, formula, solved examples like $CO_2$, $NO_2^-$, and $SO_4^{2-}$, along with MCQs for JEE and NEET exam preparation. Click the print button to download study material and notes.
What is Formal Charge ?
In polyatomic ions, the net charge belongs to the ion as a whole and not to a single atom. However, it is possible to assign a formal charge (FC) on each atom to track the distribution of electrons in a molecule or ion.
Definition
The formal charge of an atom is defined as:
The difference between the number of valence electrons in a free atom and the number of electrons assigned to it in a Lewis structure.
Formula
The formal charge can be expressed as:
$$
\text{FC} = V \:- \: L \:- \: \frac{1}{2}S
$$
Where:
- $V$ = total valence electrons in the free atom
- $L$ = total number of nonbonding (lone pair) electrons. Each lone pair has two non bonding pair of electrons.
- $S$ = total number of bonding (shared) electrons
Key Assumption : We can count the number of electrons assigned to an atom on this assumption and then compare the number with the number of electrons in the free atom.
- If the atom has more electrons in the molecule than in the free or neutral atom, then the atom has a negative formal charge (FC).
- If the atom has less electrons in the molecule than in the free or neutral atom, then the atom has a positive formal charge (FC).
Example 1: What is the formal charge on all the atoms of Ozone (O3) molecule ?
Consider the ozone molecule:
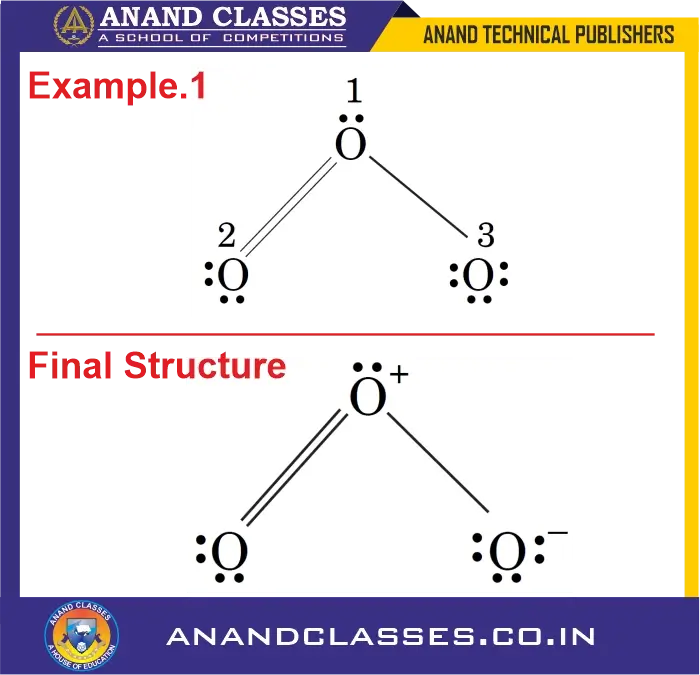
- Central O atom (O1): The central O atom marked 1 has 6 valence electrons, one lone pair (2 electrons) and three bonds (or 6 bonding electrons).
- $V=6$, $L=2$, $S=6$
$$
\text{FC}_{O1} = 6 – 2 – \frac{6}{2} = +1
$$
- $V=6$, $L=2$, $S=6$
- Terminal O atom double bonded (O2): The end O atom marked 2 has 6 valence electrons, two lone pairs (4 electrons) and two bonds (4 bonding electrons).
- $V=6$, $L=4$, $S=4$
$$
\text{FC}_{O2} = 6 – 4 – \frac{4}{2} = 0
$$
- $V=6$, $L=4$, $S=4$
- Terminal O atom single bonded (O3): The end O atom marked 3 has 6 valence electrons, three lone pairs (6 electrons) and one bond (2 bonding electrons).
- $V=6$, $L=6$, $S=2$
$$
\text{FC}_{O3} = 6 – 6 – \frac{2}{2} = -1
$$
- $V=6$, $L=6$, $S=2$
Observation: It may be noted that formal charges do not indicate real charge separation within the molecules. These only help in keeping track of the valence electrons in the molecule. The formal charges help in the selection of the lowest energy structure from a number of possible Lewis structures for a given molecule or ion. In general, the lowest energy structure is the one, which has lowest formal charges on the atoms.
Example 2: What is the formal charge on all the atoms of Phosgene (COCl2) molecule ?
Consider the phosgene molecule:
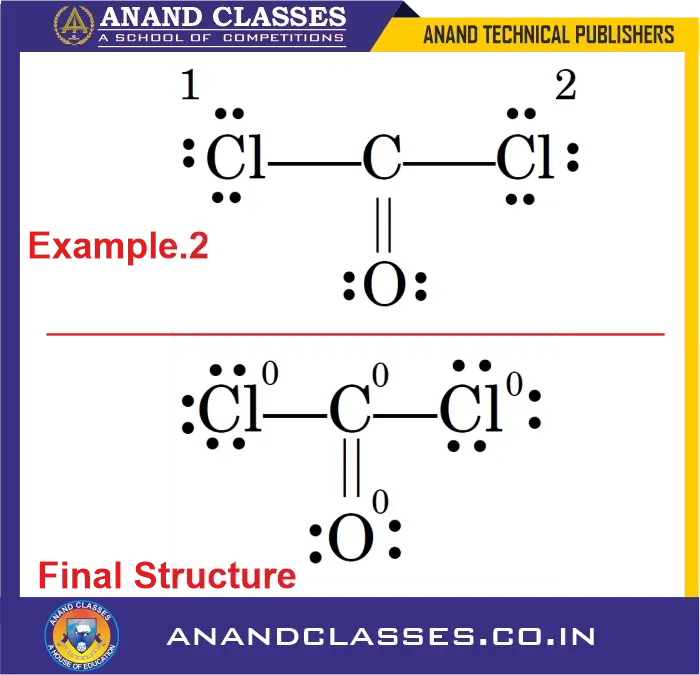
- Cl1: $V=7$, $L=6$, $S=2$
$$
\text{FC}_{Cl1} = 7 – 6 – \frac{2}{2} = 0
$$ - Cl2: $V=7$, $L=6$, $S=2$
$$
\text{FC}_{Cl2} = 7 – 6 – \frac{2}{2} = 0
$$ - O atom: $V=6$, $L=4$, $S=4$
$$
\text{FC}_{O} = 6 – 4 – \frac{4}{2} = 0
$$ - C atom: $V=4$, $L=0$, $S=8$
$$
\text{FC}_{C} = 4 – 0 – \frac{8}{2} = 0
$$
All atoms have formal charge zero, indicating a stable Lewis structure.
Example 3: What is the formal charge on all the atoms of Nitrite Ion (NO2–) ?
Consider the Nitrite Ion and Carbonate Ion :
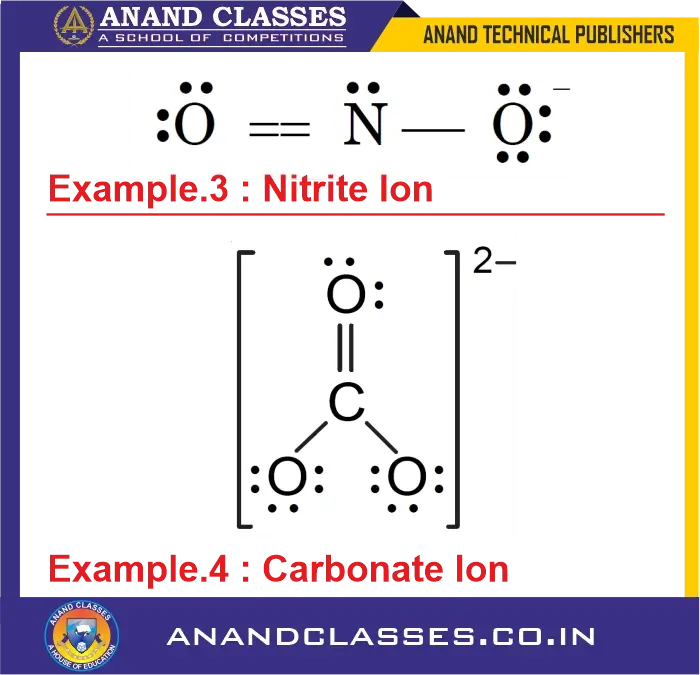
Lewis structure: $O=N-O^-$
- N atom: $V=5$, $L=2$, $S=6$
$$
\text{FC}_{N} = 5 – 2 – \frac{6}{2} = 0
$$ - Double-bonded O: $V=6$, $L=4$, $S=4$
$$
\text{FC} = 6 – 4 – \frac{4}{2} = 0
$$ - Single-bonded O: $V=6$, $L=6$, $S=2$
$$
\text{FC} = 6 – 6 – \frac{2}{2} = -1
$$
Example 4: What is the formal charge on all the atoms of Carbonate Ion (CO32-) ?
- C atom: $V=4$, $L=0$, $S=8$
$$
\text{FC}_C = 4 – 0 – \frac{8}{2} = 0
$$ - Double-bonded O: $V=6$, $L=4$, $S=4$
$$
\text{FC}_O = 6 – 4 – \frac{4}{2} = 0
$$ - Single-bonded O: $V=6$, $L=6$, $S=2$
$$
\text{FC}_O = 6 – 6 – \frac{2}{2} = -1
$$
Example 5: What is the formal charge on Cl atom in Perchloric Acid (HClO4) ?
Consider the Perchloric Acid :
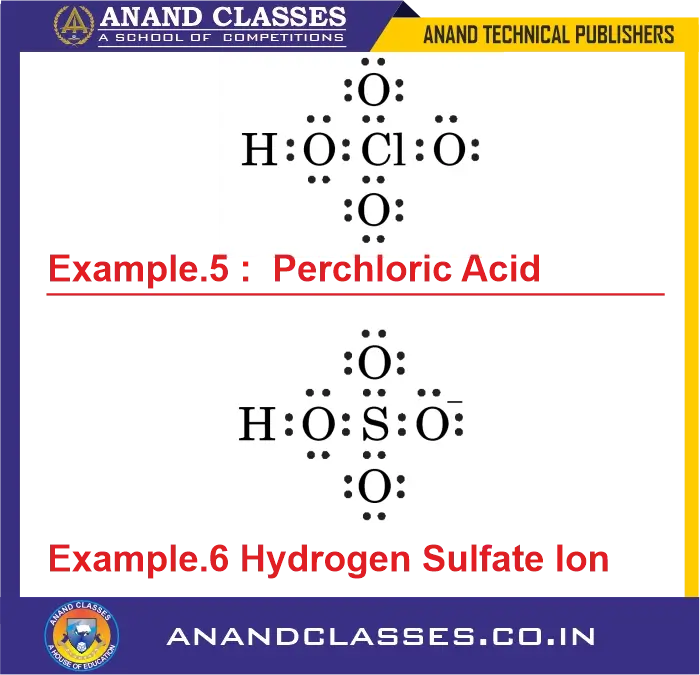
- Cl atom: $V=7$, $L=0$, $S=8$
$$
\text{FC}_{Cl} = 7 – 0 – \frac{8}{2} = +3
$$
Example 6: What is the formal charge on S atom in Hydrogen Sulfate Ion (HSO4–) ?
Consider the Hydrogen Sulfate Ion :
- S atom: $V=6$, $L=0$, $S=8$
$$
\text{FC}_S = 6 – 0 – \frac{8}{2} = +2
$$
Short Answer Conceptual Types Questions (SAT) on Formal Charge
Q1. What is formal charge?
A: Formal charge is the hypothetical charge assigned to an atom in a molecule or polyatomic ion assuming equal sharing of electrons in bonds. It helps track electron distribution and identify the most stable Lewis structure.
Q2. How is formal charge calculated?
A:
$$
\text{FC} = V – L – \frac{1}{2}S
$$
Where $V$ = valence electrons, $L$ = lone pair electrons, $S$ = shared electrons.
Q3. Does formal charge represent real charge on an atom?
A: No. It is only a bookkeeping tool to determine electron distribution and stability.
Q4. Why is formal charge important?
A: Structures with the lowest formal charges are generally more stable and closer to the real molecule.
Multiple Choice Questions (MCQs) With Answers and Explanation on Formal charge
Q1. What is the formal charge on the central O in $O_3$?
a) 0
b) +1
c) –1
d) +2
Answer: b) +1
Q2. Which atom in $CO_3^{2-}$ carries a negative formal charge?
a) C
b) Double-bonded O
c) Single-bonded O
d) All atoms
Answer: c) Single-bonded O
Q3. The sum of formal charges in a neutral molecule is:
a) Always +1
b) Always –1
c) Zero
d) Depends on bonds
Answer: c) Zero
Q4. In HClO4, the formal charge on Cl is:
a) +1
b) +2
c) +3
d) 0
Answer: c) +3
Assertion Reason Type Questions With Answers and Explanation on Formal Charge
A1. Assertion: The most stable Lewis structure has formal charges closest to zero.
Reason: Formal charge indicates actual charge on the atom.
a) Both A and R are true, and R is correct explanation of A
b) Both A and R are true, but R is not correct explanation of A
c) A is true, R is false
d) A is false, R is true
Answer: c) A is true, R is false
A2. Assertion: Ozone has a positive formal charge on the central O atom.
Reason: Central O shares electrons unequally with terminal O atoms.
a) Both A and R are true, and R is correct explanation of A
b) Both A and R are true, but R is not correct explanation of A
c) A is true, R is false
d) A is false, R is true
Answer: a) Both A and R are true, and R is correct explanation of A
Case Study based on Formal Charge
Case Study: Nitrite Ion ($NO_2^-$)
Lewis structure: $O=N-O^-$
Task 1: Calculate formal charges
- N atom: $5 – 2 – \frac{6}{2} = 0$
- Double-bonded O: $6 – 4 – \frac{4}{2} = 0$
- Single-bonded O: $6 – 6 – \frac{2}{2} = -1$
Task 2: Determine the most stable resonance structure
- The resonance structure that delocalizes the negative charge and keeps formal charges closest to zero is most stable.
Conclusion: Formal charge analysis predicts electron distribution, stability, and reactivity of ions and molecules.
Key Points
- The lowest-energy Lewis structure has the lowest formal charges.
- Formal charge is a tool for bookkeeping electrons and does not indicate actual charge separation.
- Helps predict reactivity and stability of molecules and ions.


