Anand Classes provides complete Class 11 Chemistry notes and study material for JEE and NEET on Ionic or Electrovalent Bond, explaining its formation, properties, and applications with detailed theory, solved examples, MCQs, assertion-reason questions, and case study-based problems to help students master chemical bonding concepts effectively. Click the print button to download study material and notes.
What is Ionic or Electrovalent Bond ?
When a bond is formed by the transference of one or more valence electrons of one atom to the other atom so as to complete their outermost octets and acquire stable nearest noble gas configurations, the bond formed is called electrovalent bond or ionic bond.
In this type of bonding, one atom completes its octet and acquires noble gas configuration by losing the electrons and the other by gaining the electrons. The atom which loses the electrons acquires a positive charge, and the other which gains the electrons acquires a negative charge. These two oppositely charged ions, thus formed, come closer due to electrostatic forces of attraction and form an ionic bond.
Thus, the electrostatic force of attraction which holds the oppositely charged ions together is known as ionic bond or electrovalent bond. The compounds containing ionic or electrovalent bonds are called ionic or electrovalent compounds.
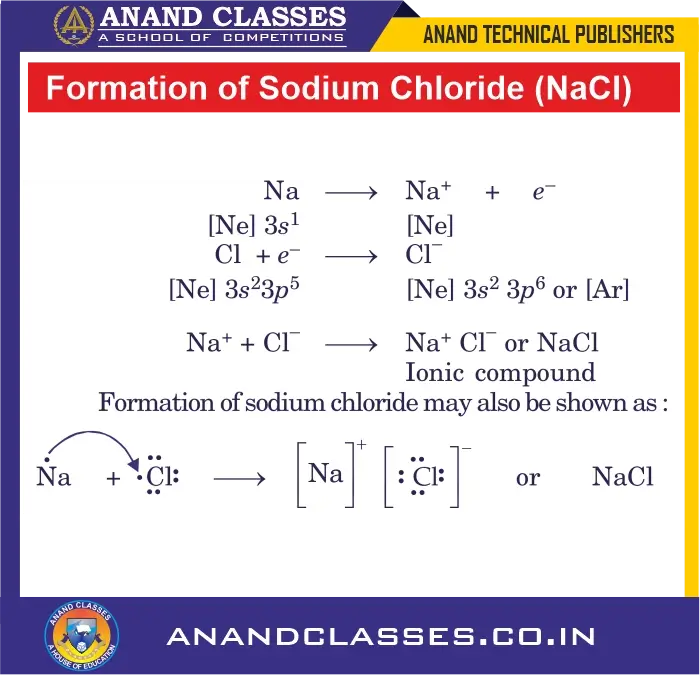
Formation of Sodium Chloride (NaCl) – An Ionic Compound
Let us illustrate the formation of ionic bond by considering the example of sodium chloride.
Sodium atom: $1s^2 2s^2 2p^6 3s^1$ has only one electron in the valence shell, and by losing this electron, it can acquire the stable electronic configuration of neon: $1s^2 2s^2 2p^6$.
Chlorine atom: $1s^2 2s^2 2p^6 3s^2 3p^5$ has seven electrons in its valence shell and needs only one electron to complete its octet. Thus, both atoms can complete their octets if sodium atom gives one electron to chlorine atom. This tendency is responsible for bonding between sodium and chlorine atoms.
Therefore, sodium gives one electron and becomes positively charged Na$^{\mathbf{+}}$ ion, while chlorine takes up the electron and becomes negatively charged Cl$^{\mathbf{-}}$ ion. These two ions are then held together by electrostatic forces of attraction which constitute the ionic bond.
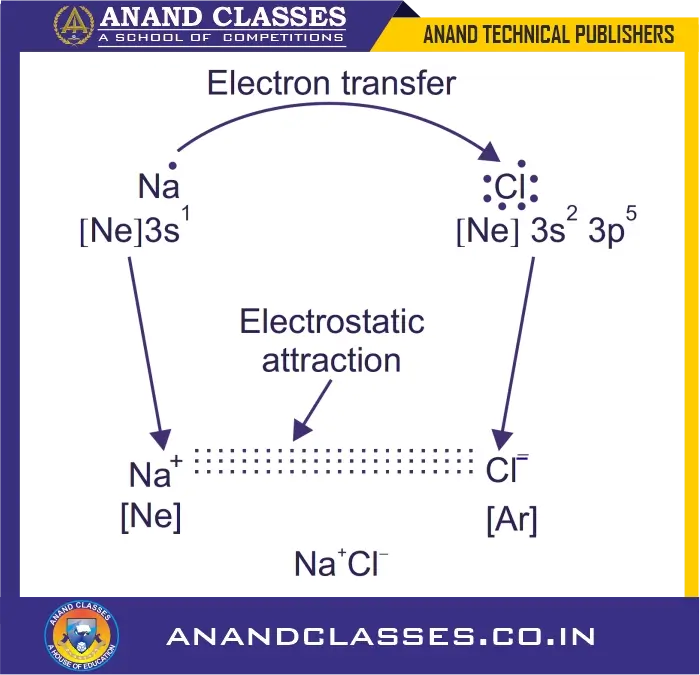
Lewis structure representation:
$$
\mathbf{Na} \;\mathbf{\longrightarrow}\; \mathbf{Na^{\mathbf{+}}} + e^- \quad : \quad [\mathbf{Ne}] 3s^1 \;\mathbf{\longrightarrow}\; [\mathbf{Ne}]
$$
$$
\mathbf{Cl} + e^- \;\mathbf{\longrightarrow}\; \mathbf{Cl^{\mathbf{-}}} \quad : \quad [\mathbf{Ne}] 3s^2 3p^5 \;\mathbf{\longrightarrow}\; [\mathbf{Ne}] 3s^2 3p^6 = [\mathbf{Ar}]
$$
$$
\mathbf{Na^{\mathbf{+}}} + \mathbf{Cl^{\mathbf{-}}} \;\mathbf{\longrightarrow}\; \mathbf{NaCl}
$$
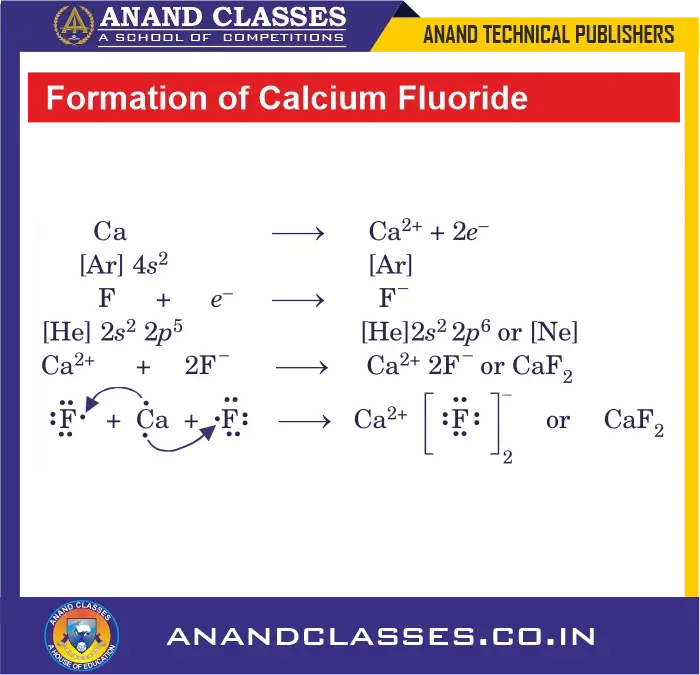
Formation of Calcium Fluoride (CaF2) – An Ionic Compound
Electronic configurations:
- Ca ($Z = 20$): $[\mathbf{Ar}] 4s^2$
- F ($Z = 9$): $[\mathbf{He}] 2s^2 2p^5$
In the formation of calcium fluoride, calcium loses both valence electrons to two fluorine atoms, each of which needs one electron. This results in the formation of Ca$^{\mathbf{2+}}$ ion and two F$^{\mathbf{-}}$ ions. Each of these ions acquires noble gas configuration. One Ca$^{\mathbf{2+}}$ and two F$^{\mathbf{-}}$ ions form bonds to give calcium fluoride.
Stepwise representation:
$$
\mathbf{Ca} \;\mathbf{\longrightarrow}\; \mathbf{Ca^{\mathbf{2+}}} + 2e^- \quad : \quad [\mathbf{Ar}] 4s^2 \;\mathbf{\longrightarrow}\; [\mathbf{Ar}]
$$
$$
\mathbf{F} + e^- \;\mathbf{\longrightarrow}\; \mathbf{F^{\mathbf{-}}} \quad : \quad [\mathbf{He}] 2s^2 2p^5 \;\mathbf{\longrightarrow}\; [\mathbf{He}] 2s^2 2p^6 = [\mathbf{Ne}]
$$
$$
\mathbf{Ca^{\mathbf{2+}}} + 2\mathbf{F^{\mathbf{-}}} \;\mathbf{\longrightarrow}\; \mathbf{CaF_2}
$$
What is Electrovalence or Electrovalency ?
In the formation of electrovalent bond, the number of electrons lost or gained by an atom is called its electrovalence or electrovalency. It is also equal to the number of unit charges on the ion.
For example :
- Sodium is assigned a positive electrovalence of one
- Calcium is assigned a positive electrovalence of two
- Chlorine and fluorine both are assigned a negative electrovalence of one
Atoms that readily lose electrons are called electropositive, while those which readily gain electrons are called electronegative.
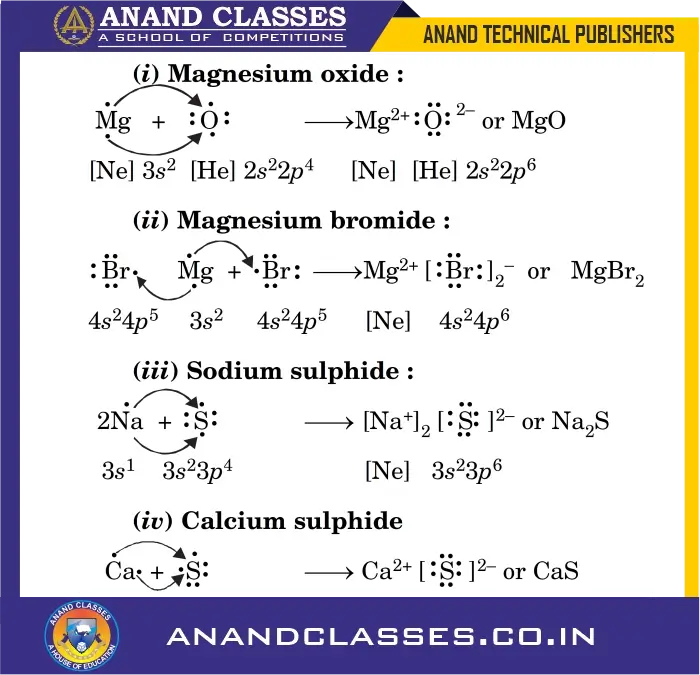
More Examples of Ionic Bond Formation
(i) Magnesium Oxide:
$$
\mathbf{Mg} + \mathbf{O} \;\mathbf{\longrightarrow}\; \mathbf{Mg^{\mathbf{2+}}} + \mathbf{O^{\mathbf{2-}}} \;\mathbf{\longrightarrow}\; \mathbf{MgO}
$$
(ii) Magnesium Bromide:
$$
\mathbf{Mg} + 2\mathbf{Br} \;\mathbf{\longrightarrow}\; \mathbf{Mg^{\mathbf{2+}}} + 2\mathbf{Br^{\mathbf{-}}} \;\mathbf{\longrightarrow}\; \mathbf{MgBr_2}
$$
(iii) Sodium Sulphide:
$$
2\mathbf{Na} + \mathbf{S} \;\mathbf{\longrightarrow}\; 2\mathbf{Na^{\mathbf{+}}} + \mathbf{S^{\mathbf{2-}}} \;\mathbf{\longrightarrow}\; \mathbf{Na_2S}
$$
(iv) Calcium Sulphide:
$$
\mathbf{Ca} + \mathbf{S} \;\mathbf{\longrightarrow}\; \mathbf{Ca^{\mathbf{2+}}} + \mathbf{S^{\mathbf{2-}}} \;\mathbf{\longrightarrow}\; \mathbf{CaS}
$$
Short Answer Conceptual Types Questions (SAT) on Ionic/Electrovalent Bond
Q1: What is an ionic bond?
A1: An ionic bond is formed by the transfer of one or more valence electrons from one atom to another, resulting in oppositely charged ions held together by electrostatic forces.
Q2: Which atom becomes positively charged in ionic bonding?
A2: The atom that loses electrons becomes positively charged (cation).
Q3: Which atom becomes negatively charged in ionic bonding?
A3: The atom that gains electrons becomes negatively charged (anion).
Q4: What is electrovalence?
A4: Electrovalence is the number of electrons lost or gained by an atom in forming an ionic bond. It equals the unit charge on the ion.
Q5: Give an example of an ionic compound.
A5: Examples include NaCl, CaF$_2$, MgO, Na$_2$S.
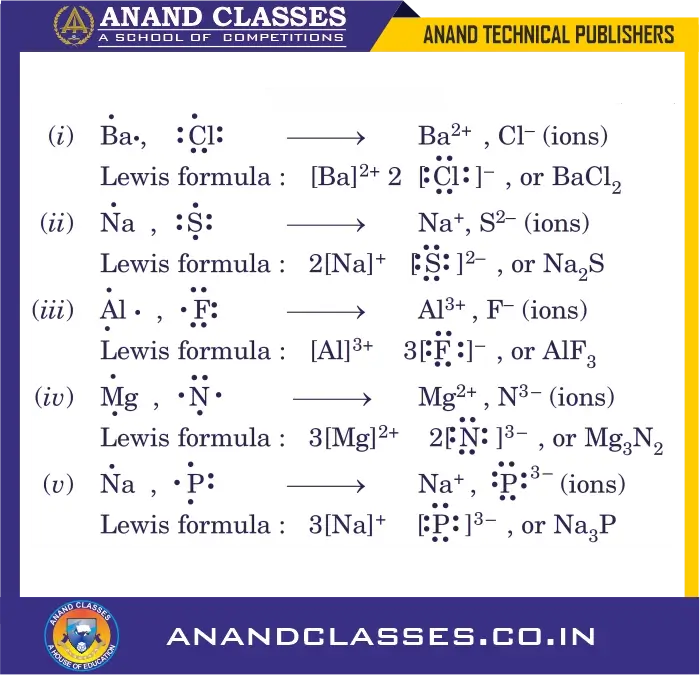
Q6: Give the Lewis structures and empirical formulae for the ionic compounds formed between the following pairs of elements :
(i) Ba, Cl (ii) Na, S (iii) Al, F (iv) Mg, N (v) Na, P
A6: From the charges on the ions, the number of positive and negative ions in the compound can be found out. This gives the empirical formula (E.F.)
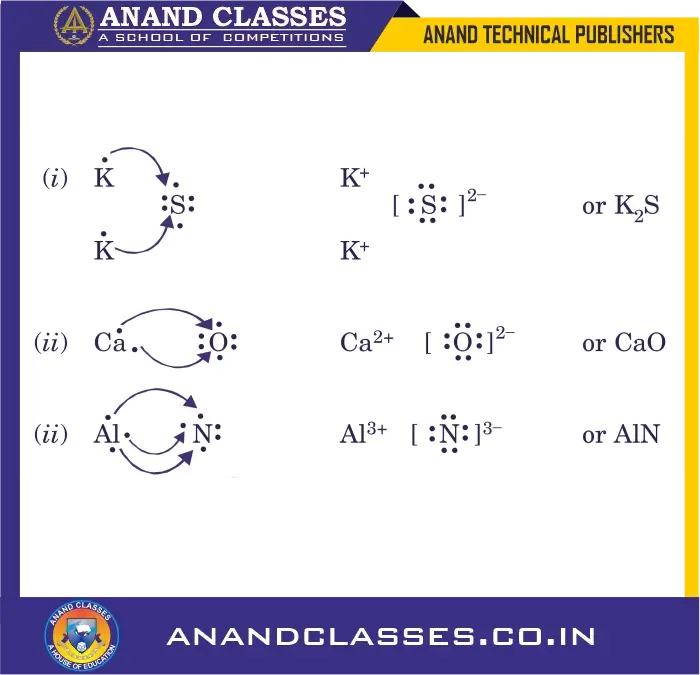
Q7: Use Lewis symbols to show electron transfer between the following atoms to form cations and anions :
(i) K and S (ii) Ca and O (iii) Al and N
A7: Lewis symbols to show electron transfer between the following atoms to form cations and anions :
Multiple Choice Questions (MCQs) With Answers and Explanation on Ionic/Electrovalent Bond
Q1: In NaCl formation, sodium atom:
(a) Gains one electron
(b) Loses one electron
(c) Shares one electron
(d) None of these
Answer: (b) Loses one electron
Q2: The number of electrons transferred in CaF2 formation is:
(a) 1
(b) 2
(c) 3
(d) 4
Answer: (b) 2
Q3: The compound MgBr2 is:
(a) Covalent
(b) Ionic
(c) Metallic
(d) Hydrogen bonded
Answer: (b) Ionic
Q4: Electropositive elements are:
(a) Elements that gain electrons
(b) Elements that lose electrons
(c) Noble gases
(d) Halogens
Answer: (b) Elements that lose electrons
Q5: In Na2S, the charge on sodium is:
(a) +1
(b) +2
(c) -1
(d) -2
Answer: (a) +1
Assertion Reason Type Questions With Answers and Explanation on Ionic/Electrovalent Bond
Q1:
Assertion (A): Sodium chloride is an ionic compound.
Reason (R): Sodium transfers one electron to chlorine forming Na+ and Cl– ions.
Options:
(a) Both A and R are true, R is correct explanation of A
(b) Both A and R are true, R is not correct explanation of A
(c) A is true, R is false
(d) A is false, R is true
Answer: (a)
Q2:
Assertion (A): Fluorine becomes negatively charged in CaF2.
Reason (R): Fluorine gains two electrons from calcium.
Options:
(a) Both A and R are true, R is correct explanation of A
(b) Both A and R are true, R is not correct explanation of A
(c) A is true, R is false
(d) A is false, R is true
Answer: (c)
Q3:
Assertion (A): Electrovalency of chlorine is -1.
Reason (R): Chlorine has seven valence electrons and needs one electron to complete octet.
Options:
(a) Both A and R are true, R is correct explanation of A
(b) Both A and R are true, R is not correct explanation of A
(c) A is true, R is false
(d) A is false, R is true
Answer: (a)
Case Study / Application-based Questions on Ionic/Electrovalent Bond
A student mixes solid calcium and fluorine in a reaction vessel. The products are collected.
Questions:
- Write the chemical formula of the product.
- Indicate the charge on calcium and fluorine ions.
- Represent the ionic bond formation using Lewis symbols.
Answers:
- CaF$_2$
- Calcium: Ca$^{\mathbf{2+}}$, Fluorine: F$^{\mathbf{-}}$
$$
\mathbf{Ca} \;\mathbf{\longrightarrow}\; \mathbf{Ca^{\mathbf{2+}}} + 2e^- \quad ; \quad 2\mathbf{F} + 2e^- \;\mathbf{\longrightarrow}\; 2\mathbf{F^{\mathbf{-}}}
$$
Case 2:
Magnesium reacts with oxygen to form a compound.
Questions:
- Identify the compound formed.
- Determine the electrovalence of magnesium and oxygen.
- Represent the ionic bond formation.
Answers:
- MgO
- Magnesium: +2, Oxygen: -2
$$
\mathbf{Mg} + \mathbf{O} \;\mathbf{\longrightarrow}\; \mathbf{Mg^{\mathbf{2+}}} + \mathbf{O^{\mathbf{2-}}} \;\mathbf{\longrightarrow}\; \mathbf{MgO}
$$


