Anand Classes explains the Van der Waals radius is a measure of the size of an atom when it is not bonded to another atom but held together by weak Van der Waals forces in the solid state. Unlike covalent radius, which is measured for bonded atoms, Van der Waals radius is determined for non-bonded atoms in neighbouring molecules. This concept is especially important for noble gases, which do not form covalent bonds and therefore have their atomic radii expressed in terms of Van der Waals radii.
Why Van der Waals Radius is Needed ?
- Atomic radius values for most elements are covalent radii because they are measured in covalently bonded molecules.
- Noble gases (e.g., Neon, Argon) do not form covalent molecules like Ne₂ or Ar₂ under normal conditions.
- In their solid state, noble gas atoms are held together by weak van der Waals forces, not by covalent bonds.
- Hence, for noble gases and similar non-bonded atoms, we measure the Van der Waals radius instead of covalent radius.
Named after Johannes Diderik van der Waals, who studied weak intermolecular forces.
Definition of Van der Waals Radius
Van der Waals Radius is:
Van der Waals radius is defined as half of the internuclear separation of two non-bonded atoms of the same element on their closest possible approach. The term is used for non- metals (covalent compound)
and noble gases.or
It is one–half of the distance between the nuclei of two adjacent atoms belonging to two neighbouring molecules of an element in the solid state.
This is applicable when the atoms are not bonded covalently but are held together by weak Van der Waals forces.
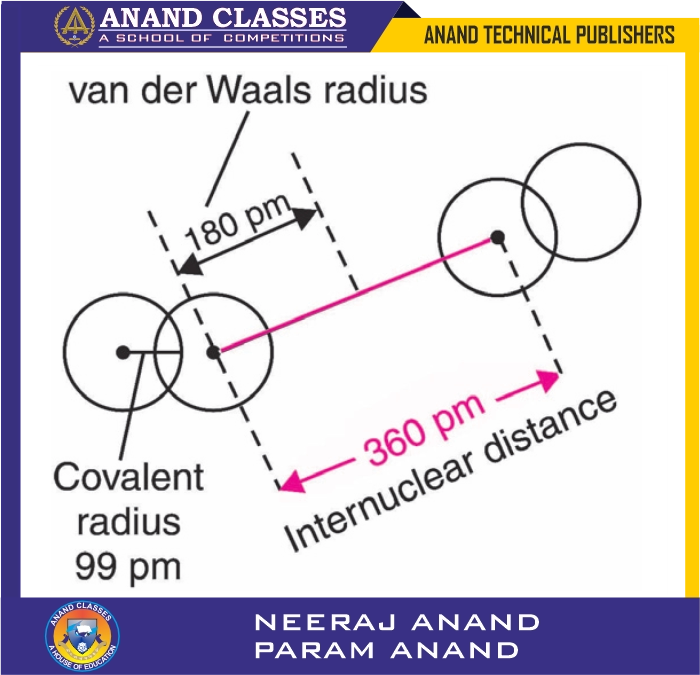
Examples
- Chlorine (Cl2) in solid state:
- Internuclear distance = 360 pm (3.6 Å)
- Van der Waals radius = 360/2 = 180 pm (1.80 Å)
- Hydrogen (H2) in solid state:
- Internuclear distance = 240 pm
- Van der Waals radius = 240/2 = 120 pm (1.2 Å)
Why Noble Gases Show Van der Waals Radii ?
- Noble gases (except Xe and Kr) do not form covalent bonds because of their complete octets.
- Their atomic radius cannot be measured as covalent radius.
- Instead, we measure Van der Waals radius, since these atoms exist as discrete monoatomic species and are held together only by Van der Waals forces in the solid state.
- Also known as Inert Gas Radii.
Why Van der Waals Radii is Greater than Covalent Radii ?
The covalent radius is always smaller than the corresponding van der Waal’s radius. This is because of the fact that in the formation of a chemical bond, the two atoms have to come closer to each other. This also explains why covalent bonds are much stronger than the van der Waal’s forces. It is important to note that since the noble gases ordinarily do not form any covalent bond, in crystals of noble gases, no chemical forces are operating between the atoms. Hence the van der Waal’s forces are the only attractive forces in these cases.
In other words, the van der Waal’s radii constitute the atomic radii of noble gases and since van der Waal’s radii are larger than covalent radii, atomic radii of noble gases are largest in their respective periods (anomaly).
Atoms held by van der Waals forces are farther apart than covalently bonded atoms → van der Waals radii > covalent radii.
Periodic Trends in Van der Waals Radii
(a) Across a Period
- Decreases from left to right due to increase in nuclear charge.
| Element | N | O | F |
|---|---|---|---|
| Radius (pm) | 150 | 140 | 135 |
(b) Down a Group (Halogens)
- Increases due to addition of new shells.
| Element | F | Cl | Br | I |
|---|---|---|---|---|
| Radius (pm) | 135 | 180 | 195 | 215 |
(c) Down a Group (Noble Gases)
- Increases down the group.
| Element | He | Ne | Ar | Kr | Xe |
|---|---|---|---|---|---|
| Radius (pm) | 120 | 160 | 191 | 200 | 220 |
Difference Between Covalent and Van der Waals Radii
| Feature | Covalent Radius | Van der Waals Radius |
|---|---|---|
| Nature of Bond | For covalently bonded atoms | For non-bonded atoms held by van der Waals forces |
| Magnitude | Smaller | Larger |
| Measurement State | Molecule in gaseous or solid covalent state | Solid state with weak intermolecular forces |
| Example | H–H bond distance in H₂ | Cl–Cl distance in solid Cl₂ |
FAQs on Van der Waals Radius
Q1. What is the Van der Waals radius?
Ans: The Van der Waals radius is half of the internuclear distance between the nuclei of two adjacent atoms belonging to neighbouring molecules in the solid state. It is measured when atoms are held together by weak Van der Waals forces, not by covalent bonds.
Q2. Why is the Van der Waals radius larger than the covalent radius?
Ans: Van der Waals forces are weak, so atoms are held at larger distances compared to covalent bonds. This makes the Van der Waals radius always larger than the covalent radius.
Q3. Why do noble gases have Van der Waals radii instead of covalent radii?
Ans: Noble gases (except Xe and Kr) generally do not form chemical compounds or covalent bonds. Their atomic size is therefore measured using the Van der Waals radius, as in the solid state they are held together only by Van der Waals forces.
Q4. How does the Van der Waals radius vary in a period and a group?
Ans:
Across a period: It decreases from left to right due to increasing nuclear charge.
Down a group: It increases due to the addition of extra electron shells.
Q5. Can you give an example of calculating Van der Waals radius?
Ans: Yes. The internuclear distance between adjacent chlorine atoms in solid state is 360 pm.
Van der Waals radius = 360/2 = 180 pm or 1.80 Å.
Q6. What are some examples of Van der Waals radii for noble gases?
Ans: He: 120 pm, Ne: 160 pm, Ar: 191 pm, Kr: 200 pm, Xe: 220 pm
Do You Know? – Van der Waals Radius
- 💡 The concept of Van der Waals radius is named after Johannes Diderik van der Waals, who first explained the weak forces of attraction between non-bonded atoms and molecules.
- 🛑 No chemical bond needed! Van der Waals radius is measured when atoms are not chemically bonded, unlike covalent or metallic radii.
- 🏆 Van der Waals forces are responsible for the condensation of noble gases into liquids and solids at very low temperatures.
- 🧊 In solid noble gases, atoms are held only by Van der Waals forces, making their crystals extremely soft and with low melting points.
- 📏 The Van der Waals radius is always larger than the covalent radius of the same atom. For example, Cl has a covalent radius of 99 pm but a Van der Waals radius of 180 pm.
- 🧬 Geckos can walk on walls because of Van der Waals forces between their foot pads and the wall surface!
- 📚 Van der Waals radius plays a major role in molecular modeling and drug design, as it helps in predicting how molecules pack together in crystals.
📚 Buy Study Material & Join Our Coaching
For premium study materials specially designed for JEE, NEET, NDA, and CBSE/ICSE Classes, visit our official study material portal:
👉 https://anandclasses.net.in/
To enroll in our offline or online coaching programs, visit our coaching center website:
👉 https://anandclasses.co.in/
📞 Call us directly at: +91-94631-38669
💬 WhatsApp Us Instantly
Need quick assistance or want to inquire about classes and materials?
📲 Click below to chat instantly on WhatsApp:
👉 Chat on WhatsApp
🎥 Watch Video Lectures
Get access to high-quality video lessons, concept explainers, and revision tips by subscribing to our official YouTube channel:
👉 Neeraj Anand Classes – YouTube Channel


