Anand Classes provides comprehensive Class 11 Chemistry notes on Valence Bond Theory (VBT) with a clear explanation of why the H2 molecule exists but He₂ does not form. Learn how the overlapping of atomic orbitals leads to bond formation in H2 due to energy stabilization, while in He2, repulsion prevents stable bond formation. These notes cover the concept of bond formation, types of overlap, limitations of VBT, and include solved Q&A, MCQs, and exam-based practice questions for NEET, JEE, and CBSE board preparation. Click the print button to download study material and notes.
What is Valence Bond Theory (VBT) ?
The valence bond theory was put forward by Heitler and London in 1927. It was later improved and developed by L. Pauling and J. C. Slater in 1931.
The valence bond theory is based on the knowledge of atomic orbitals, electronic configurations of elements, overlap criteria of atomic orbitals and stability of molecule.
The basic assumptions of this theory are :
- Atoms do not lose their identity even after the formation of the molecule.
- The bond is formed due to the interaction of only the valence electrons as the two atoms come close to each other. The inner electrons do not participate in the bond formation.
- During the formation of bond, only the valence electrons from each bonded atom lose their identity. The other electrons remain unaffected.
- The stability of bond is accounted by the fact that the formation of bond is accompanied by release of energy. The molecule has minimum energy at a certain distance between the atoms known as internuclear distance. Larger the decrease in energy, stronger will be the bond formed.
To understand the concept more clearly, let us consider the formation of H2 molecule.
How Does Valence Bond Theory Explain formation H2 Molecule?
Consider two hydrogen atoms (A and B) approaching each other having nuclei HA and HB and the corresponding electrons eA and eB respectively.
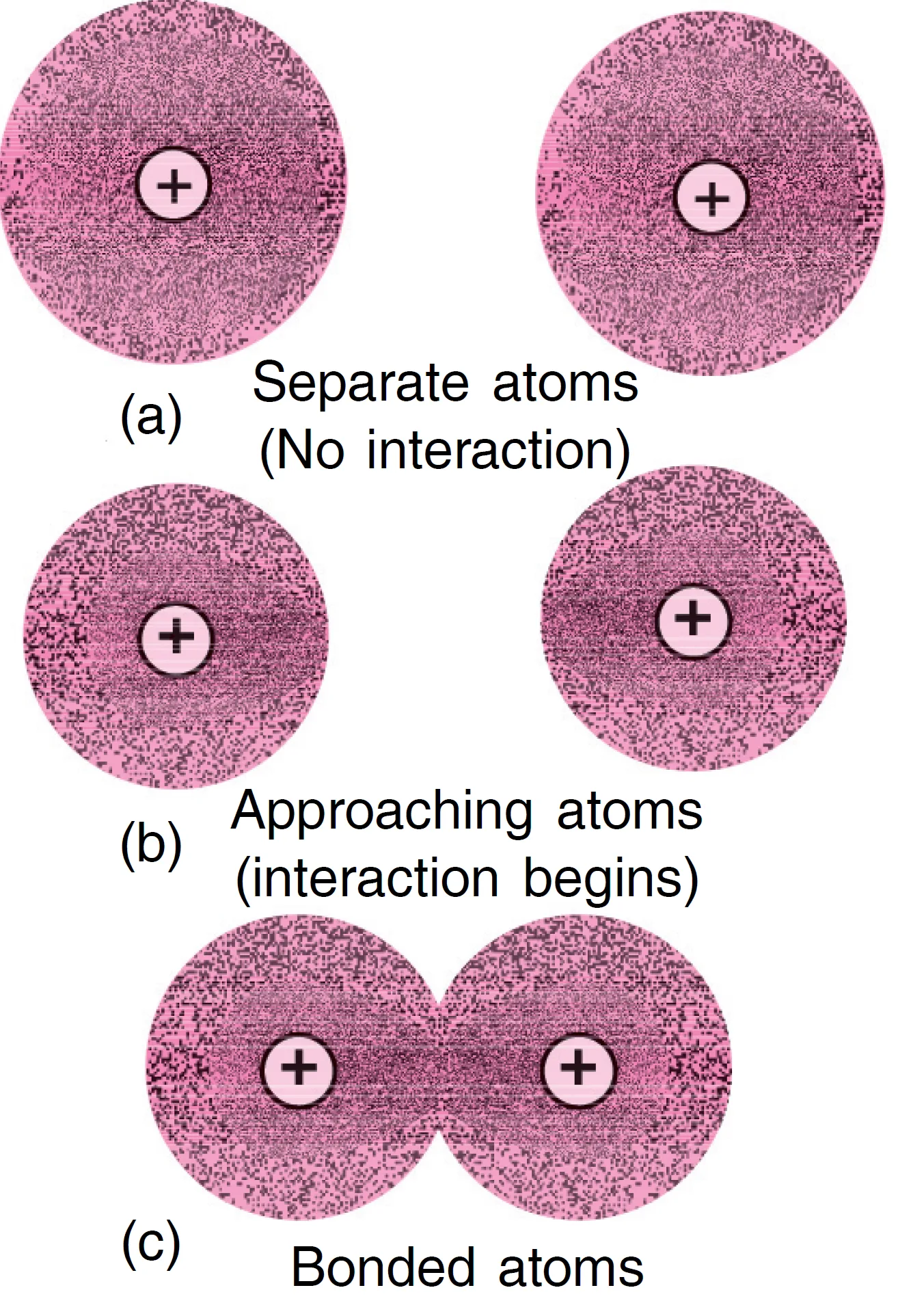
When the two atoms are at large distances, no interaction between the two atoms takes place. At this stage, the total energy of the system = sum of energies of the individual atoms.
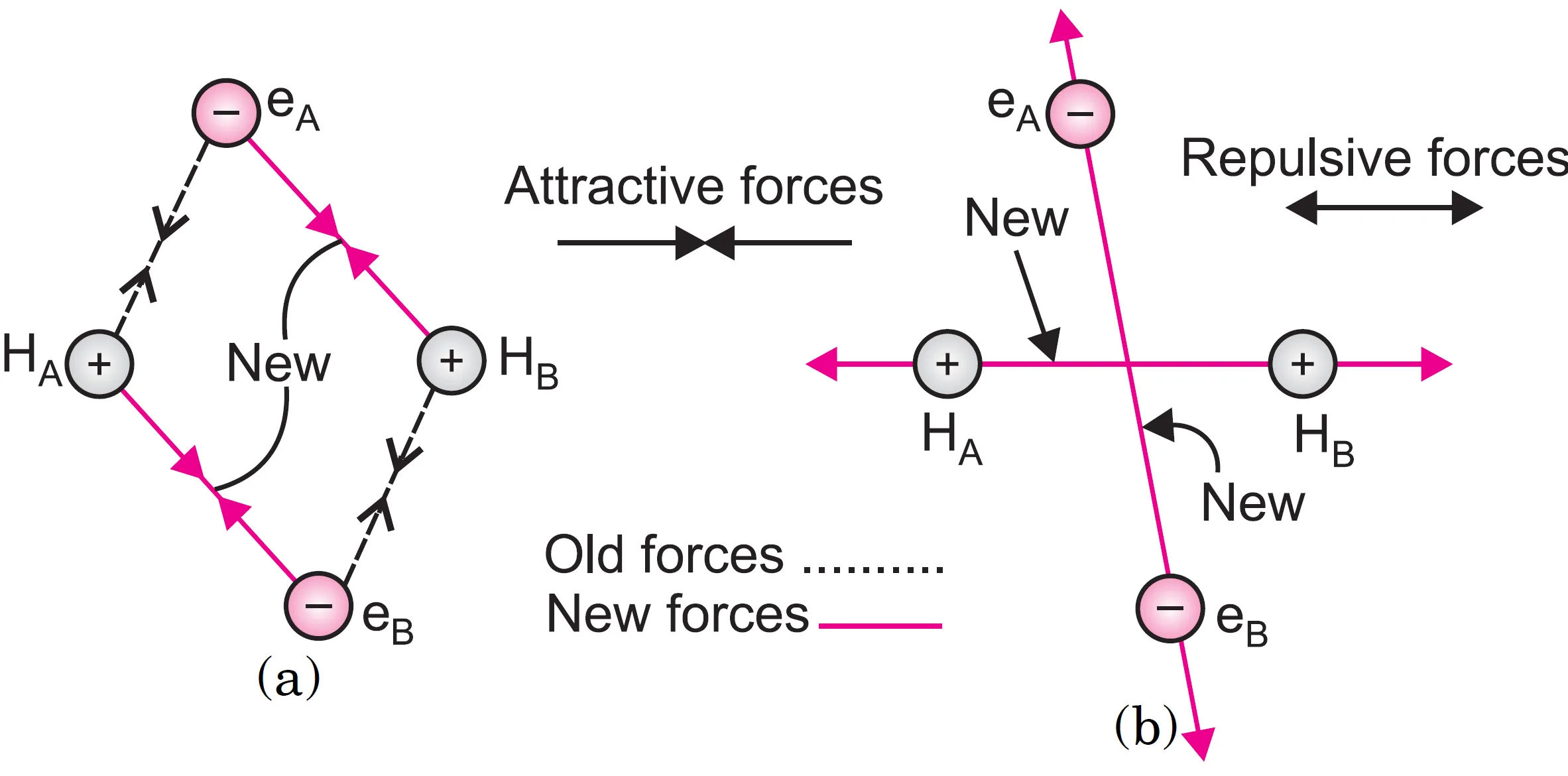
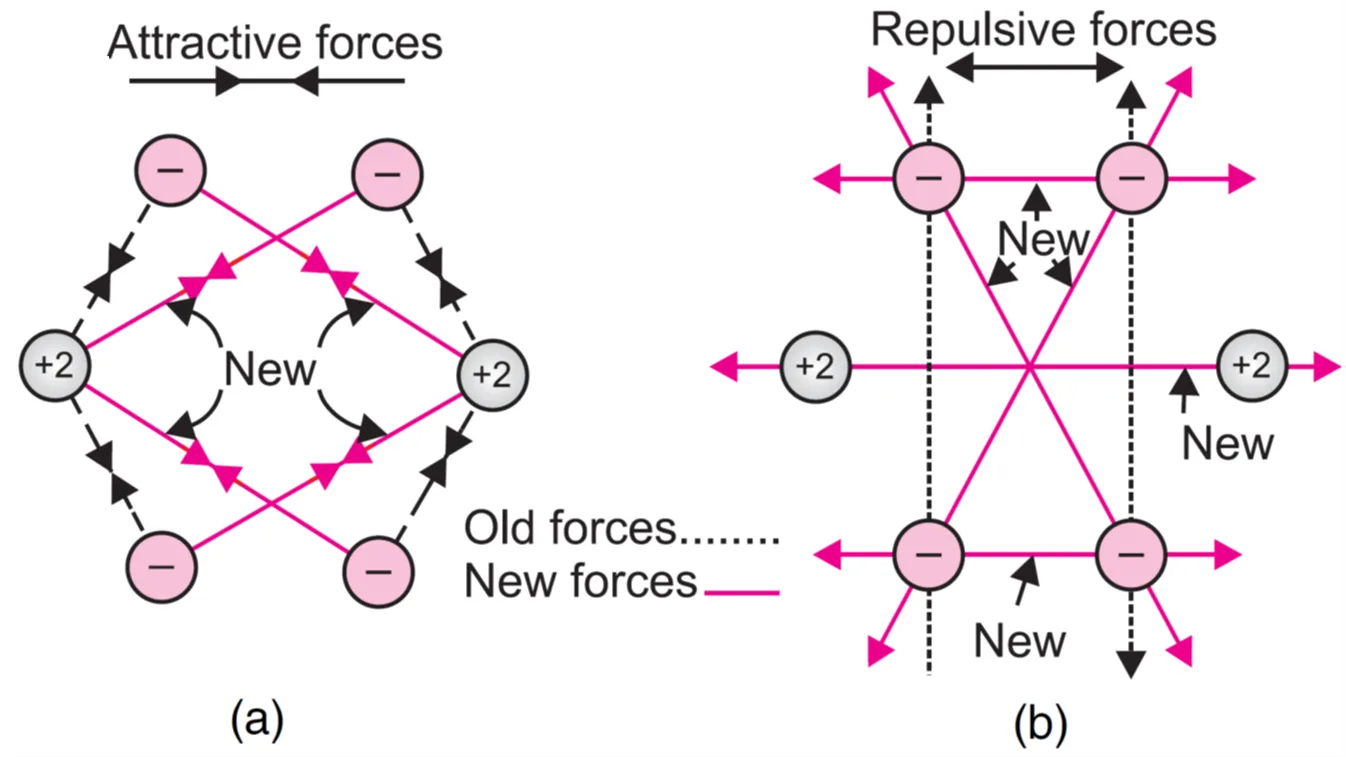
When the two atoms come closer, new attractive and repulsive forces begin to operate. Besides the attraction of the nucleus of one atom for its own electrons i.e. HA– eA and HB– eB, the following attractive and repulsive forces start operating :
Attractive forces:
(i) Nucleus–electron attraction within the atom: Attraction of the nucleus of one atom for its own electrons i.e. HA– eA and HB– eB
(ii) Cross-attraction: Attractive forces operate between electron of atom A (eA) and nucleus of B (HB) and electron of atom B (eB) and nucleus of A (HA). These two new attractive forces are shown in Figure (a).
formation of H2 molecule
Repulsive forces:
(i) Electron–electron repulsion: Repulsive forces are present between electrons of both atoms i.e. eA – eB.
(ii) Nucleus–nucleus repulsion: Repulsive forces are present between nuclei of both atoms i.e. HA – HB.
These two forces are shown in Figure(b).
Now we know that attractive forces tend to bring the atoms closer while repulsive forces tend to push them apart.
It has been observed experimentally that the magnitude of the new attractive forces is more than the new repulsive forces. As a result, the two atoms approach each other shown in Figure (a) and the potential energy of the system decreases. This situation has been shown in Figure (b) where the two atoms are close together.
As the two atoms come closer and closer, the system becomes more and more stable due to decrease of energy. Ultimately, a stage is reached where the total forces of attraction balance the forces of repulsion and the system acquires minimum energy. This is shown in Figure (c). At this stage, the two hydrogen atoms are said to be bonded together to form a stable molecule and the distance (r0) between the atoms is known as bond length.
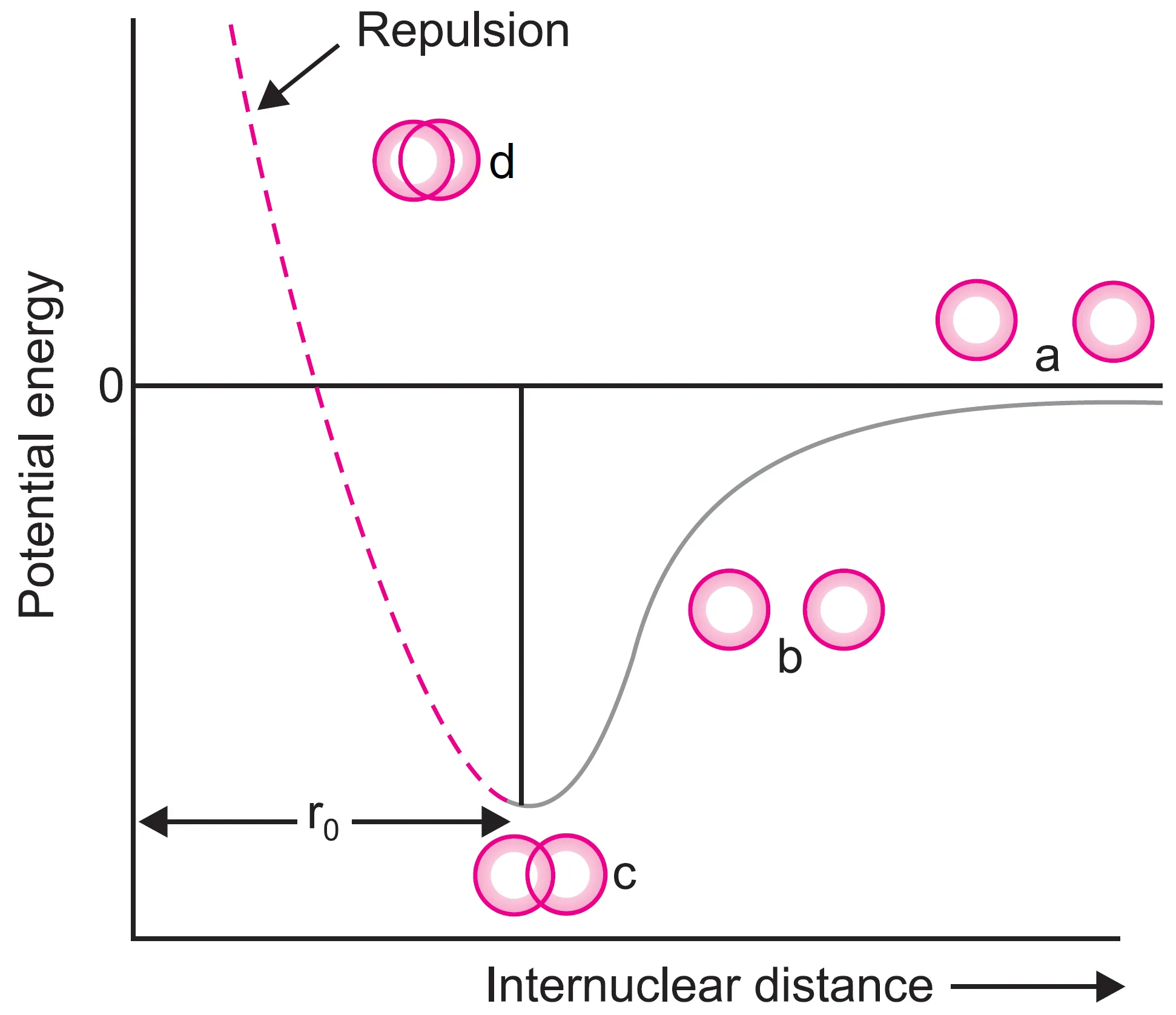
For hydrogen molecule, the distance between two hydrogen atoms corresponding to minimum energy is 74 pm. The potential energy of the system when two atoms are brought closer and closer is represented in Figure. This is called potential energy diagram.
atoms are brought closer and closer.
In this diagram, when the two atoms are for apart (stage a), there is no attractive or repulsive interactions between them and the potential energy of the system (isolated atoms) is assumed to be zero.
Thus, when the bond is formed, energy is released and therefore, the hydrogen molecule is more stable than the individual hydrogen atoms. That is
Bond energy of H2 :
$$H + H \longrightarrow H_2 + 435.8 \text{kJ mol}^{-1}$$
This energy corresponding to minimum in the curve is called bond energy.
Conversely, when one mole of H2 molecules is dissociated to hydrogen atoms, 435.8 kJ of energy is needed.
$$H_2 (g) + 435.8 \text{kJ mol}^{-1} \longrightarrow H (g) + H (g)$$
It may be remembered that the two hydrogen atoms cannot be brought closer than 74 pm because then the repulsive forces will become large and the potential energy would rise [stage(d) shown by dotted line in Figure].
Summary
Since attractive forces > repulsive forces, the atoms move closer, lowering the potential energy of the system.
- A stage is reached where attraction balances repulsion → system has minimum energy.
- The distance between the two H atoms at this stage is called bond length (ro). For hydrogen, bond length = 74 pm.
The potential energy diagram shows:
- At infinite distance → potential energy = 0.
- As atoms approach → potential energy decreases.
- At 74 pm → minimum potential energy → stable molecule.
- If atoms come closer than 74 pm → repulsion increases sharply, potential energy rises.
Bond energy of H2 :
$$H + H \longrightarrow H_2 + 435.8 \text{kJ mol}^{-1}$$
Thus, H2 is more stable than individual H atoms. Conversely, dissociating H₂ requires 435.8 kJ mol-1:
$$H_2 (g) + 435.8 \text{kJ mol}^{-1} \longrightarrow H (g) + H (g)$$
It may be remembered that the two hydrogen atoms cannot be brought closer than 74 pm because then the repulsive forces become large and the potential energy rises.
Why is Helium Molecule (He2) Not Formed ?
Each helium atom has two electrons in its 1s-orbital. There are two attractive forces between the nucleus and the electrons of each atom. These are shown by dotted lines in Figure (a).
helium atoms
When two helium atoms approach each other:
- 4 new attractive forces arise (nucleus–electron).
- 5 new repulsive forces arise (nucleus–nucleus and electron–electron).
Since repulsive forces > attractive forces, the potential energy increases, leading to instability.
Thus, He2 represents an unstable state, and a chemical bond is not formed between helium atoms. Hence, He2 molecule is not formed.
Short Answer Conceptual Type Questions (SAT) with Answers
Q1. Who proposed the Valence Bond Theory? Who improved it later?
Answer: VBT was proposed by Heitler and London (1927) and later improved by L. Pauling and J. C. Slater (1931).
Q2. Write any three assumptions of Valence Bond Theory.
Answer:
- Atoms do not lose their identity after bond formation.
- Only valence electrons take part in bond formation.
- Bond formation occurs when attractive forces > repulsive forces between atoms.
Q3. Why is bond formation always accompanied by release of energy according to VBT?
Answer: Because when a bond forms, the system achieves minimum potential energy, making the molecule stable, and the excess energy is released.
Q4. Define internuclear distance. What is its value in H₂ molecule?
Answer: The internuclear distance is the distance between nuclei of two bonded atoms. In H₂ molecule, it is 74 pm.
Q5. State the bond energy of H2 molecule.
Answer: The bond energy of H2 is 435.8 kJ mol⁻¹.
Q6. Why is the He2 molecule not formed?
Answer: In He2, repulsive forces dominate (5 repulsions vs 4 attractions), making it unstable, so He₂ is not formed.
Q7. Which electrons take part in bond formation according to Valence Bond Theory?
Answer: Only valence electrons participate in bond formation.
Multiple Choice Questions (MCQs) with Answers and Explanations
Q1. Which of the following is NOT an assumption of Valence Bond Theory?
(a) Atoms lose their identity after bond formation
(b) Only valence electrons take part in bond formation
(c) Bond formation is accompanied by release of energy
(d) Molecules are stable at minimum potential energy
Answer: (a)
Explanation: Atoms retain their identity after bond formation.
Q2. The bond length of H2 molecule according to Valence Bond Theory is:
(a) 54 pm
(b) 74 pm
(c) 104 pm
(d) 124 pm
Answer: (b)
Explanation: H2 has a bond length of 74 pm, where attraction and repulsion balance.
Q3. The bond energy of H2 molecule is:
(a) 225 kJ mol⁻¹
(b) 335 kJ mol⁻¹
(c) 435.8 kJ mol⁻¹
(d) 535.8 kJ mol⁻¹
Answer: (c)
Explanation: H₂ bond energy is 435.8 kJ mol⁻¹.
Q4. Why is He₂ not formed according to VBT?
(a) Attractive forces > repulsive forces
(b) Repulsive forces > attractive forces
(c) Overlap of p-orbitals is not possible
(d) Bond energy is very high
Answer: (b)
Explanation: In He₂, repulsions dominate attractions, making it unstable.
Assertion–Reason Type Questions with Answers and Explanations
Q1.
Assertion (A): H2 molecule is stable and has a bond length of 74 pm.
Reason (R): In H2, attractive forces are greater than repulsive forces at 74 pm internuclear distance.
(a) Both A and R are true, and R is the correct explanation of A.
(b) Both A and R are true, but R is not the correct explanation of A.
(c) A is true, R is false.
(d) A is false, R is true.
Answer: (a)
Explanation: At 74 pm, attraction = repulsion, giving stability and minimum energy.
Q2.
Assertion (A): He2 molecule does not exist.
Reason (R): In He2, repulsive forces are greater than attractive forces.
(a) Both A and R are true, and R is the correct explanation of A.
(b) Both A and R are true, but R is not the correct explanation of A.
(c) A is true, R is false.
(d) A is false, R is true.
Answer: (a)
Explanation: Repulsive forces > attractive forces in He₂, hence unstable.
Q3.
Assertion (A): Bond formation is always accompanied by release of energy.
Reason (R): Molecules have minimum potential energy at infinite internuclear distance.
(a) Both A and R are true, and R is the correct explanation of A.
(b) Both A and R are true, but R is not the correct explanation of A.
(c) A is true, R is false.
(d) A is false, R is true.
Answer: (c)
Explanation: Molecules have minimum potential energy at finite bond length, not at infinity.
Case Study Based Questions
Passage:
Valence Bond Theory (VBT) explains bond formation through orbital overlap. Atoms retain their identity after bonding and only valence electrons participate. A bond is formed when attractive forces outweigh repulsive forces, releasing energy and making the system stable. In H₂ molecule, the bond length is 74 pm and the bond energy is 435.8 kJ mol⁻¹. The potential energy curve shows a minimum at bond length. He2 molecule, however, is not formed as repulsions dominate attractions, making it unstable.
Questions:
- Who improved VBT in 1931?
- What is the bond length of H2 molecule?
- Why is bond formation accompanied by release of energy?
- Why is He2 molecule not stable?
- Which electrons are involved in bond formation according to VBT?
Answers:
- L. Pauling and J. C. Slater.
- 74 pm.
- Because the system attains minimum potential energy at bond length.
- Because in He₂, repulsive forces exceed attractive forces.
- Valence electrons.