Anand Classes provides NCERT Exemplar Solutions for questions 49 to 55 (Long Type Questions) from the chapter Classification of Elements and Periodicity in Properties for Class 11 Chemistry. These solutions are carefully explained to help students develop a deeper understanding of periodic properties, element classification, and related chemical principles with detailed steps, examples, and diagrams to enhance learning and boost performance in exams. Click the print button to download study material and notes.
Q. 49 Discuss the factors affecting electron gain enthalpy and the trend in its variation in the periodic table.
Ans. Electron gain enthalpy ($\Delta_{eg}H$) of an element is the energy released when an electron is added to the valence shell of an isolated gaseous atom.
$$ A(g) + e^- \;\;\longrightarrow\;\; A^-(g) \quad \Delta_{eg}H < 0 $$
Factors affecting electron gain enthalpy
- Effective nuclear charge (Zeff):
- As effective nuclear charge increases, the attraction of the nucleus towards the incoming electron increases.
- Hence, electron gain enthalpy becomes more negative.
- Size of the atom:
- Larger the atomic size, weaker the attraction of the nucleus towards the added electron.
- Hence, electron gain enthalpy becomes less negative with increase in size.
- Type of subshell (orbital):
- Closer the subshell is to the nucleus, easier it is to add an electron.
- General order (for same $n$ value):
$$ s > p > d > f $$
- Nature of electronic configuration:
- Atoms with half-filled or fully-filled orbitals are particularly stable.
- Addition of an electron to such configurations is not energetically favorable, so $\Delta_{eg}H$ becomes less negative (or may even be positive).
Variation in the periodic table (Across a period (left to right)) :
- Effective nuclear charge increases and atomic size decreases.
- Hence, electron gain enthalpy becomes more negative across a period.
- Example:
$$ \Delta_{eg}H(\text{Cl}) < \Delta_{eg}H(\text{S}) < \Delta_{eg}H(\text{P}) < \Delta_{eg}H(\text{Na}) $$
Variation in the periodic table (Down a group (top to bottom))
- Atomic size increases, shielding effect increases.
- The added electron feels less attraction from the nucleus.
- Hence, electron gain enthalpy becomes less negative down the group.
- Exception:
- $\Delta_{eg}H$ of O and F is less negative than those of S and Cl respectively.
- Reason: In O and F, the incoming electron enters the smaller $n=2$ shell where electron-electron repulsions are stronger.
- In S and Cl, the added electron enters the larger $n=3$ shell where repulsions are less.
Final Key Points:
- Across a period → $\Delta_{eg}H$ becomes more negative.
- Down a group → $\Delta_{eg}H$ becomes less negative.
- Exceptions: O, F have less negative $\Delta_{eg}H$ than S, Cl due to higher electron-electron repulsions in smaller orbitals.
Electron gain enthalpy depends on Zeff, atomic size, subshell type, and electronic configuration.
Q. 50 Define ionisation enthalpy. Discuss the factors affecting ionisation enthalpy of the elements and its trends in the periodic table.
Ans. Ionisation enthalpy is defined as the minimum amount of energy required to remove the most loosely bound electron from an isolated gaseous atom to convert it into a gaseous cation.
It is represented by $\Delta_i H$.
Factors affecting ionisation enthalpy of the elements
(i) Nuclear charge
Ionisation enthalpy increases with increase in nuclear charge. This is because a higher nuclear charge attracts the outer electrons more strongly, making it harder to remove them.
For example, in the 2nd period:
| Element (Period 2) | Li | Be | B | C | N | O | F | Ne |
|---|---|---|---|---|---|---|---|---|
| Nuclear charge | +3 | +4 | +5 | +6 | +7 | +8 | +9 | +10 |
| First ionisation enthalpy (kJ mol$^{-1}$) | 520 | 899 | 801 | 1086 | 1402 | 1314 | 1681 | 2080 |
Thus, ionisation enthalpy generally increases across a period due to increasing nuclear charge.
(ii) Atomic size (radius)
Ionisation enthalpy decreases with increase in atomic size. As the distance of the valence electron from the nucleus increases, the nucleus attracts it less strongly.
Hence, less energy is required to remove it.
For alkali metals:
| Alkali metals (Group 1) | Li | Na | K | Rb | Cs |
|---|---|---|---|---|---|
| First ionisation enthalpy (kJ mol$^{-1}$) | 520 | 496 | 419 | 403 | 374 |
So, ionisation enthalpy decreases down the group.
(iii) Penetration effect of electrons
Electrons in orbitals with higher penetration are held more strongly by the nucleus, so ionisation enthalpy increases with penetration.
Penetration order: $s > p > d > f$
For example:
Mg (1s$^2$ 2s$^2$ 2p$^6$ 3s$^2$) → removal of an $s$ electron
Al (1s$^2$ 2s$^2$ 2p$^6$ 3s$^2$ 3p$^1$) → removal of a $p$ electron
Since $s$ electrons penetrate more than $p$, Al has lower ionisation enthalpy than Mg.
(iv) Shielding or screening effect
The greater the shielding effect of inner electrons, the weaker the attraction between the nucleus and valence electrons. Hence, ionisation enthalpy decreases with increasing shielding.
(v) Electron configuration (stability)
Atoms with half-filled or completely filled orbitals are more stable, so removal of an electron requires more energy.
Examples:
Be (1s$^2$ 2s$^2$) has higher ionisation enthalpy than B (1s$^2$ 2s$^2$ 2p$^1$).
N (1s$^2$ 2s$^2$ 2p$^3$) has higher ionisation enthalpy than O (1s$^2$ 2s$^2$ 2p$^4$).
Trends in the periodic table
- Across a period: Ionisation enthalpy increases due to increasing nuclear charge and decreasing atomic size.
- Down a group: Ionisation enthalpy decreases due to increasing atomic size and shielding effect, which outweigh the increase in nuclear charge.
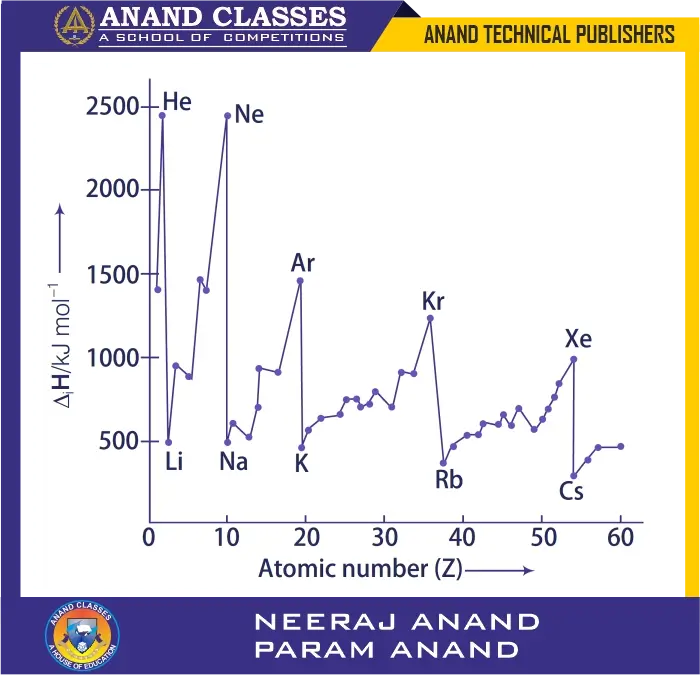
Q. 51 Justify the given statement with suitable examples – “the properties of the elements are a periodic function of their atomic numbers”.
Ans. The periodic law states that the physical and chemical properties of elements are a periodic function of their atomic numbers. This means that when the elements are arranged in order of increasing atomic number, their properties repeat at regular intervals.
The cause of periodicity is the repetition of similar outer electronic configurations after regular intervals of atomic numbers.
Example 1: Group 1 elements (Alkali metals)
All the alkali metals have similar outer electronic configuration $ns^1$.
- Lithium (Z = 3): $1s^2 2s^1$
- Sodium (Z = 11): $1s^2 2s^2 2p^6 3s^1$
- Potassium (Z = 19): $[Ar] 4s^1$
- Rubidium (Z = 37): $[Kr] 5s^1$
- Cesium (Z = 55): $[Xe] 6s^1$
- Francium (Z = 87): $[Rn] 7s^1$
Since they all have one electron in the outermost s-orbital, they exhibit similar properties:
- They are soft, highly reactive metals.
- They form basic oxides ($M_2O$), whose basic strength increases down the group.
- They form unipositive ions ($M^+$) by losing one electron.
Example 2: Group 17 elements (Halogens)
All halogens have similar outer electronic configuration $ns^2 np^5$.
- Fluorine (Z = 9): $1s^2 2s^2 2p^5$
- Chlorine (Z = 17): $[Ne] 3s^2 3p^5$
- Bromine (Z = 35): $[Ar] 3d^{10} 4s^2 4p^5$
- Iodine (Z = 53): $[Kr] 4d^{10} 5s^2 5p^5$
- Astatine (Z = 85): $[Xe] 4f^{14} 5d^{10} 6s^2 6p^5$
Since they all have seven valence electrons, their properties are similar:
- They are non-metals.
- They exist as diatomic molecules ($X_2$).
- They form uninegative ions ($X^-$).
- They all form volatile covalent halides with metals.
Conclusion:
Because of the periodic repetition of outer electronic configuration, elements in the same group (like alkali metals or halogens) exhibit similar physical and chemical properties. Hence, the properties of elements are truly a periodic function of their atomic numbers.
Q. 52 Write down the outermost electronic configurations of alkali metals. How will you justify their placement in group 1 of the periodic table?
Ans. All the elements of group IA (or group 1), i.e., alkali metals, have the similar outermost electronic configuration $ns^1$, where $n$ refers to the number of principal shell. This means they all have one electron in the outermost s-orbital.
Their outer electronic configurations are:
| Symbol | Atomic number | Electronic configuration |
|---|---|---|
| Li | 3 | $1s^2 , 2s^1$ or [He]$,2s^1$ |
| Na | 11 | $1s^2 , 2s^2 , 2p^6 , 3s^1$ or [Ne]$,3s^1$ |
| K | 19 | $[Ar],4s^1$ |
| Rb | 37 | $[Kr],5s^1$ |
| Cs | 55 | $[Xe],6s^1$ |
| Fr | 87 | $[Rn],7s^1$ |
Justification of placement in Group 1:
- Since all alkali metals have one electron in the outermost shell ($ns^1$), they exhibit similar chemical properties.
- They readily lose this single valence electron to form unipositive ions ($M^+$).
- They all form strongly basic oxides and hydroxides.
- Their metallic and chemical reactivity increases down the group as the atomic size increases and ionisation enthalpy decreases.
Hence, due to the similarity in their outer electronic configuration ($ns^1$), all these elements are placed together in Group 1 of the periodic table.
Q. 53 Write the drawbacks in Mendeleef’s periodic table that led to its modification.
Ans. The main drawbacks of Mendeleef’s periodic table are:
(i) Anomalous grouping of elements
Some elements having similar properties were placed in different groups, whereas some elements having dissimilar properties were placed in the same group.
- Example: Alkali metals (Li, Na, K, etc., Group IA) were grouped together with coinage metals (Cu, Ag, Au, Group IB) even though their properties are quite different.
- Similarly, chemically similar elements such as Cu (IB group) and Hg (IIB group) were placed in different groups.
(ii) Anomalous order of atomic weights
Some elements with higher atomic weights were placed before elements with lower atomic weights in order to maintain similarity in chemical properties.
- Example: Argon ($^{18}$Ar, 39.9) was placed before Potassium ($^{19}$K, 39.1).
- Similarly, Cobalt ($^{27}$Co, 58.9) was placed before Nickel ($^{28}$Ni, 58.7).
(iii) No place for isotopes
Mendeleef’s table did not accommodate isotopes. According to his classification, isotopes (which differ in atomic mass) should be placed separately, but this would break the periodic arrangement.
(iv) Uncertain position of hydrogen
The position of hydrogen was controversial since it resembled alkali metals (Group IA) as well as halogens (Group VIIA). Its proper placement was not justified.
(v) Position of Group VIII elements unclear
The elements of Group VIII were arranged in three triads without proper justification, which did not fit the periodic pattern.
(vi) Failure to explain long periods
The table could not explain the even and odd series in the long periods (IV, V, and VI).
(vii) No proper place for lanthanides and actinides
Lanthanides and actinides (discovered later) were not given positions in the main body of the table, making the arrangement incomplete.
Note: Most of these defects were later removed by the Modern Periodic Law (based on atomic number instead of atomic mass), which led to the modern periodic table.
✅ Here’s a comparison table of Mendeleef’s periodic law vs. Modern periodic law for quick revision:
| Basis | Mendeleef’s Periodic Law | Modern Periodic Law |
|---|---|---|
| Definition | Properties of elements are a periodic function of their atomic masses. | Properties of elements are a periodic function of their atomic numbers. |
| Arrangement | Elements arranged in increasing order of atomic mass. | Elements arranged in increasing order of atomic number. |
| Position of Isotopes | Isotopes could not be placed properly (since they have different masses). | Isotopes placed at the same position (same atomic number). |
| Anomalies | Some elements with higher atomic mass placed before lower mass ones (e.g., Ar–K, Co–Ni). | No such anomalies; order is strictly based on atomic number. |
| Hydrogen Position | Position was uncertain (resembled both alkali metals and halogens). | Still debatable, but more justifiable by electronic configuration. |
| Noble Gases | Noble gases were not discovered, so no place was given. | Noble gases placed in Group 18, completing the table. |
| Lanthanides & Actinides | No proper place given in the table. | Placed separately below the main body of the periodic table. |
| Justification | Explained periodicity only partly; many inconsistencies remained. | Better justified by electronic configuration and periodic trends. |
👉 Conclusion: Mendeleef’s law was the foundation, but the modern periodic law (based on atomic number) resolved anomalies and gave the periodic table its present form.
Q. 54 In what manner is the long form of periodic table better than Mendeleef’s periodic table? Explain with examples.
Ans. The long form of the periodic table is better than Mendeleef’s periodic table because it classifies the elements on the basis of the electronic configurations of their atoms, which explains their periodicity more logically and accurately.
Characteristics of the Long Form Periodic Table
(i) The table consists of 18 vertical columns (groups) and 7 horizontal rows (periods), unlike Mendeleef’s which had only 8 groups.
(ii) Groups are numbered from 1 to 18. This systematic numbering removes the confusion of subgroups (A and B) in Mendeleef’s table.
(iii) Elements are classified into well-defined families:
- Group 1 (IA) → Alkali metals (Li, Na, K, Rb, Cs, Fr)
- Group 2 (IIA) → Alkaline earth metals (Be, Mg, Ca, Sr, Ba, Ra)
- Group 17 (VIIA) → Halogens (F, Cl, Br, I, At)
- Group 18 (0) → Noble gases (He, Ne, Ar, Kr, Xe, Rn)
(iv) Transition elements are placed separately in the middle (Groups 3–12). Elements like Fe, Co, Ni or Ru, Rh, Pd form well-defined series instead of “triads” as in Mendeleef’s table.
(v) The Lanthanides (6th period) and Actinides (7th period) are placed separately at the bottom of the table, without disturbing the main framework.
(vi) Based on their electronic configuration, elements are grouped into s-, p-, d-, and f-blocks, which makes prediction of their properties systematic.
(vii) The table clearly shows a gradual change in properties (such as atomic size, ionisation enthalpy, electronegativity, metallic character) across a period and down a group.
Example:
- Alkali metals (Group 1) all have outer electronic configuration $ns^1$ and show similar properties such as forming unipositive ions ($M^+$), reacting with water to form hydroxides, etc.
- Halogens (Group 17) all have outer electronic configuration $ns^2np^5$, and hence they show similar properties such as forming diatomic molecules ($F_2, Cl_2$) and forming negative ions ($X^-$).
👉 Thus, the long form periodic table explains periodic trends on the basis of atomic number and electronic configuration, removing the defects of Mendeleef’s atomic mass-based arrangement.
✅ Here’s a comparison table of Mendeleef’s Periodic Table vs. Long Form (Modern) Periodic Table for quick revision:
| Feature | Mendeleef’s Periodic Table | Long Form (Modern) Periodic Table |
|---|---|---|
| Basis of classification | Atomic mass | Atomic number & electronic configuration |
| Numbering of groups | 8 groups (I–VIII), each with subgroups A & B | 18 groups, no subgroups (clearer arrangement) |
| Periods | 7 periods (not clearly differentiated in length) | 7 well-defined periods with increasing elements (short, long, very long) |
| Position of isotopes | Could not be explained (different masses → should be placed separately) | All isotopes placed together (same atomic number) |
| Anomalies (e.g., Ar–K, Co–Ni) | Present (heavier elements sometimes placed before lighter ones) | Removed (strictly increasing atomic number order) |
| Noble gases | Not discovered when table was proposed → no place | Placed in Group 18, completing the table |
| Transition elements | Group VIII arranged in “triads” (Fe, Co, Ni etc.), confusing | Clearly placed in d-block (Groups 3–12) |
| Lanthanides & Actinides | No separate place | Placed separately below the main table as f-block |
| Property explanation | Could not explain periodicity in terms of structure | Explained trends (atomic size, ionisation enthalpy, electronegativity) using electronic configuration |
| Hydrogen | Position uncertain (resembled both Group 1 & Group 17) | Placed in Group 1 (but still considered special) |
| Predictive power | Could predict existence of undiscovered elements (e.g., Eka-Silicon → Germanium) | More systematic and accurate, still used as the standard today |
👉 Conclusion:
The long form periodic table is superior because it is based on atomic number, explains periodic trends better, and provides a logical classification of s-, p-, d-, and f-block elements.
Q. 55 Discuss and compare the trend in ionisation enthalpy of the elements of group 1 with those of group 17 elements.
Ans. Ionisation enthalpy is the amount of energy required to remove the most loosely bound electron from an isolated gaseous atom. The values of the first ionisation enthalpies of group 1 and group 17 elements are as follows:
| Group 1 (Alkali Metals) | Ionisation Enthalpy (kJ mol⁻¹) | Group 17 (Halogens) | Ionisation Enthalpy (kJ mol⁻¹) |
|---|---|---|---|
| H | 1312 | F | 1681 |
| Li | 520 | Cl | 1255 |
| Na | 496 | Br | 1142 |
| K | 419 | I | 1009 |
| Rb | 403 | At | 917 |
| Cs | 374 | — | — |
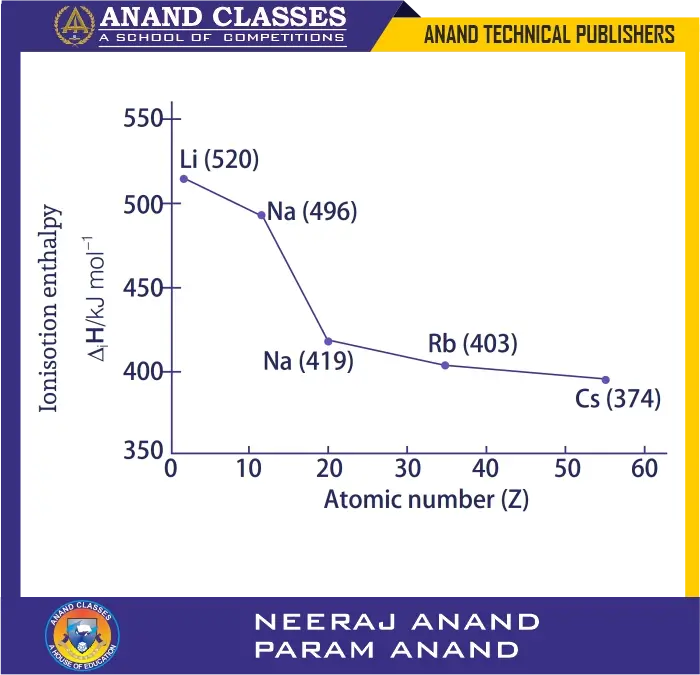
Trend in Group 1 (Alkali Metals):
- Ionisation enthalpy decreases regularly down the group.
- This is due to increase in atomic size (addition of new principal energy shell) and increase in screening effect.
- Both these factors reduce the effective nuclear attraction for valence electrons.
- Hence, the outermost electron is more easily removed in heavier alkali metals.
- Example: Li (520) → Cs (374).
Trend in Group 17 (Halogens):
- Ionisation enthalpy also decreases down the group, but the values are much higher compared to group 1 elements.
- Halogens have small atomic size and high effective nuclear charge, so they hold their valence electrons very tightly.
- Example: F (1681) → At (917).
Comparison of Group 1 vs Group 17:
- Group 1 elements have very low ionisation enthalpies, because they need to lose one electron to attain stable noble gas configuration.
- Group 17 elements have very high ionisation enthalpies, because they are just one electron short of completing their octet, so they do not easily lose electrons.
- In both groups, the values decrease down the group due to increase in atomic radius and screening effect.
- However, the absolute values are always much higher for group 17 than for group 1.
Reasoning behind the trend:
- (i) Increase in atomic size down the group → valence electron farther from the nucleus → easier removal → ionisation enthalpy decreases.
- (ii) Increase in screening effect → inner electrons shield valence electrons from nucleus → weak nuclear attraction → easier removal.
- (iii) Increase in nuclear charge → tends to increase ionisation enthalpy.
- But, the effect of atomic size and screening outweighs nuclear charge → overall decrease down the group.
Final Conclusion:
- Group 1 elements have low ionisation enthalpies and lose electrons readily → behave as strong reducing agents.
- Group 17 elements have high ionisation enthalpies and do not lose electrons easily → instead, they gain electrons and act as strong oxidising agents.
📚 Buy Study Material & Join Our Coaching
For premium study materials specially designed for JEE, NEET, NDA, and CBSE/ICSE Classes, visit our official study material portal:
👉 https://publishers.anandclasses.co.in/
For NDA Study Material, Click Here
For SSC Study Material, Click Here
To enroll in our offline or online coaching programs, visit our coaching center website:
👉 https://anandclasses.co.in/
📞 Call us directly at: +91-94631-38669
💬 WhatsApp Us Instantly
Need quick assistance or want to inquire about classes and materials?
📲 Click below to chat instantly on WhatsApp:
👉 Chat on WhatsApp
🎥 Watch Video Lectures
Get access to high-quality video lessons, concept explainers, and revision tips by subscribing to our official YouTube channel:
👉 Neeraj Anand Classes – YouTube Channel


