Anand Classes provides comprehensive Class 11 Chemistry notes on Fajan’s Rules and Degree of Polarisation explaining how ionic bonds acquire partial covalent character. Understand how factors such as the size and charge of cations, the size of anions, and electronic configuration determine the extent of polarisation in ionic compounds. These notes cover applications of Fajan’s Rules, examples like AlCl₃ and LiI, and their role in predicting bond nature. Well-structured Q&A, MCQs, and exam-focused explanations make these notes highly useful for NEET, JEE, and CBSE board preparation. Click the print button to download study material and notes.
What is Partial Covalent Character in Ionic Bonds?
Ionic and covalent bonds are two extreme types of bonds. In fact, most of the heteronuclear bonds have intermediate character. Even in the case of pure ionic compounds, there is some degree of covalent character.
When two oppositely charged ions A+ and B– are brought together, the positive ion attracts the outermost electrons of the negative ion. This results in distortion of electron clouds around the anion towards the cation. This distortion of electron cloud of the negative ion by the positive ion is called polarisation.
- If the polarisation is small, the bond is mainly ionic.
- If the degree of polarisation is large, electron cloud is drawn more from the negative ion to the positive ion. Consequently, the charges on the ions become less (negative becomes less negative and positive charge also becomes less positive because some of it is neutralised by the electron cloud of anion).
- This decreases the ionic character of the bond and favours the covalent character.
The polarisation of an anion by cation is shown below. In Fig. (a), the two ions are shown without any polarisation, assuming that the bond is completely ionic. This represents an ideal ion pair.
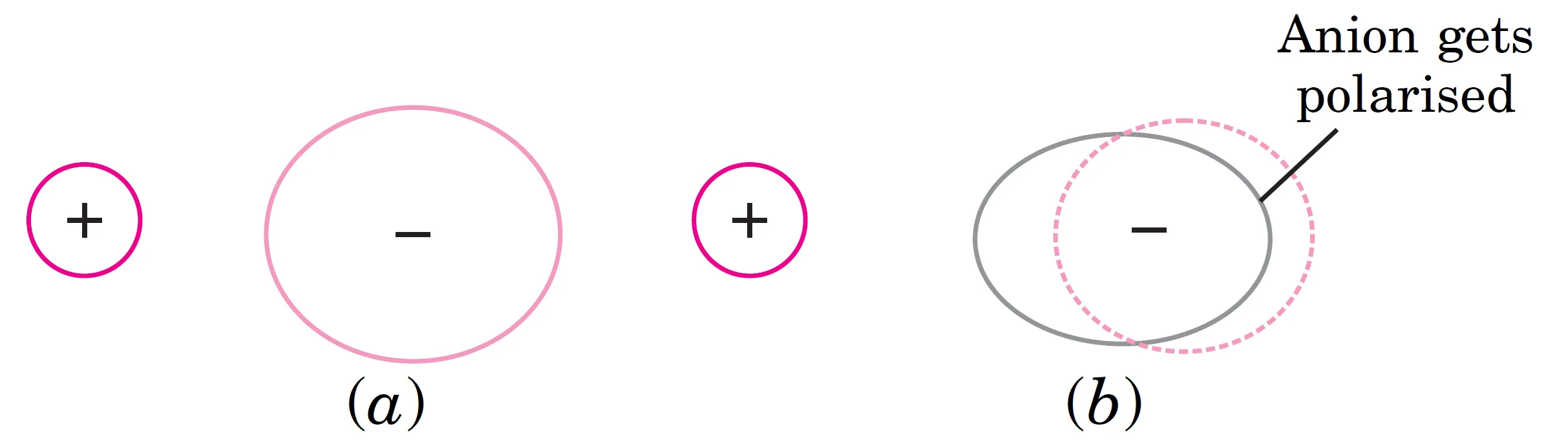
In Fig. (b) the positive ion polarises the negative ion and draws electrons towards itself. As a result of polarisation, there is decrease of positive charge on the cation and decrease of negative charge on the anion. This leads to covalent character. Obviously, if polarisation is more, larger will be covalent character of the bond.
Degree of Polarisation
The type of bond depends upon the degree of polarisation:
- If there is no polarisation, the bond is mainly ionic.
If the degree of polarisation is small, the bond will be ionic with some covalent character and - If the polarisation is more, the covalent character becomes predominent.
Summary
- No polarisation → mainly ionic bond
- Small polarisation → ionic with some covalent character
- Large polarisation → covalent character predominant
What Factors Affect the Extent of Polarisation?
The extent of polarisation depends on both the ions (Cation and Anion):
(i) The power of the cation to distort or polarise the anion
(ii) The susceptibility of the anion to get polarised by the cation
These rules are summarised in Fajan’s Rules.
How Does the Size of Cation Affect Polarisation?
Smaller the size of the cation, greater is its polarising power.
- Small cations have high electron density and distort the electron cloud of the anion to a greater extent.
- Compounds containing small cations will have more covalent character.
Example: LiCl is more covalent than NaCl and KCl.
The polarising power of a cation decreases with increase in its size.
Summary
Small size of the cation → greater polarising power.
How Does the Size of Anion Affect Polarisation?
Polarisation increases with increase in size of anion.
- The electron cloud of a bigger anion is held less firmly by its nucleus and is easily deformed towards the cation.
- Thus, larger the anion, the higher will be its polarisability and more will be covalent character in its compounds.
- Therefore, the anions such as I–, Br–, S2–, Se2–, etc. are easily polarisable and have larger tendency to induce covalent character in ionic compounds.
Example: Covalent character of lithium halides follows the order:
LiI > LiBr > LiCl > LiF
Summary
Larger the anion → higher its polarisability → more covalent character.
How Does the Charge of Cation or Anion Influence Covalent Character?
Large charge on cation or anion → stronger polarisation.
- Larger the charge on cation → greater its polarising power.
- Larger the charge on anion → greater its tendency to get polarised.
Example: Na+ and Ca2+ have almost similar ionic radii, but CaCl2 has higher covalent character than NaCl because of the higher charge on Ca2+.
| Compound | Cation Radius (Å) | Melting Point (K) |
|---|---|---|
| NaCl | Na+ : 0.95 Å | 1073 K |
| CaCl2 | Ca2+ : 0.99 Å | 1045 K |
→ CaCl2 has lower melting point, supporting its greater covalent character.
How Does the Electronic Configuration of the Cation Affect Covalent Character?
Cations with 18-electron shell configuration (pseudo inert gas configuration) cause greater polarisation than cations with 8-electron shell configuration of same size and charge.
This is due to the fact that in case of 18 electrons shell ion, there are 10 d-electrons in addition to eight s-and p–electrons. The d-electrons donot shield the nuclear charge effectively and therefore, they have increased effective nuclear charge. Consequently, these ions behave as though they are under the influence of greater nuclear charge and polarise the anion to a
greater extent. Therefore, such compounds will have more covalent character.
For example, if we compare the ions of same size; Na+ and Cu+, Na+ has eight electrons shell configuration (2s22p6) while Cu+ has 18 electrons shell configuration (3s2 3p6 3d10). The Cu+ ion polarize the anion more than Na+ ion. As a result, copper chloride, CuCl is slightly covalent and therefore, insoluble in water whereas NaCl is ionic and highly soluble in water.
The covalent character of CuCl in comparison to NaCl is also supported by its low melting point. Melting point of CuCl is 705 K while that of NaCl is 1073 K.
What Do Fajan’s Rules Predict About Covalent Character in Ionic Compounds?
According to Fajan’s rules, covalent character is favoured by:
(i) Small size of cation
(ii) Large size of anion
(iii) High charge of cation and anion
(iv) Cations with 18-electron shell configuration
Short Answer Conceptual Type Questions (SAT) on Fajan’s Rules
Q1. What is meant by polarisation of an anion?
Answer: Distortion of the electron cloud of an anion by a cation is called polarisation.
Q2. Why does $LiCl$ show more covalent character than $NaCl$?
Answer: $Li^{+}$ is smaller in size and has greater polarising power, so it distorts $Cl^{-}$ more than $Na^{+}$.
Q3. Arrange $LiF, LiCl, LiBr, LiI$ in increasing covalent character.
Answer: $LiF < LiCl < LiBr < LiI$
Explanation: Covalent character increases with increasing anion size ($F^{-} \to I^{-}$).
Q4. Why is $CaCl_{2}$ more covalent than $NaCl$ even though their cation sizes are similar?
Answer: Because $Ca^{2+}$ has higher charge than $Na^{+}$, so it has greater polarising power.
Q5. Which cation causes more polarisation: $Na^{+} (2p^{6})$ or $Cu^{+} (3d^{10})$? Why?
Answer: $Cu^{+}$ polarises more because of its 18-electron (pseudo-inert gas) configuration which increases polarising power.
Multiple Choice Questions (MCQs) on Fajan’s Rules
Q1. Which of the following has the highest covalent character?
(a) $NaCl$
(b) $KCl$
(c) $LiCl$
(d) $CsCl$
Answer: (c) $LiCl$
Explanation: Smaller cation → higher polarising power → more covalent character.
Q2. Which of the following is the correct order of covalent character?
(a) $LiF > LiCl > LiBr > LiI$
(b) $LiI > LiBr > LiCl > LiF$
(c) $LiCl > LiF > LiI > LiBr$
(d) $LiBr > LiI > LiCl > LiF$
Answer: (b) $LiI > LiBr > LiCl > LiF$
Explanation: Larger anion → higher polarisability → greater covalent character.
Q3. $CaCl_{2}$ has more covalent character than $NaCl$ because:
(a) $Ca^{2+}$ has larger size than $Na^{+}$
(b) $Ca^{2+}$ has smaller size than $Na^{+}$
(c) $Ca^{2+}$ has higher charge than $Na^{+}$
(d) Both have same polarising power
Answer: (c) $Ca^{2+}$ has higher charge than $Na^{+}$
Explanation: Higher charge on cation → stronger polarisation → more covalent.
Q4. Which type of cations show more covalent character according to Fajan’s Rules?
(a) Large, low charge
(b) Small, high charge
(c) Neutral atoms
(d) Large, high charge
Answer: (b) Small, high charge
Explanation: Small size + high charge → maximum polarising power.
Assertion-Reason Type Questions on Fajan’s Rules
Q1.
Assertion (A): $LiI$ has more covalent character than $LiF$.
Reason (R): Larger anions are more polarisable.
- (a) Both A and R are true, and R is the correct explanation of A.
- (b) Both A and R are true, but R is not the correct explanation of A.
- (c) A is true, R is false.
- (d) A is false, R is true.
Answer: (a)
Explanation: $I^{-}$ is much larger than $F^{-}$, hence more polarisable → stronger covalent character.
Q2.
Assertion (A): $CuCl$ is more covalent than $NaCl$.
Reason (R): Cations with 18-electron configuration polarise more than cations with 8-electron configuration.
- (a) Both A and R are true, and R is the correct explanation of A.
- (b) Both A and R are true, but R is not the correct explanation of A.
- (c) A is true, R is false.
- (d) A is false, R is true.
Answer: (a)
Explanation: $Cu^{+} (3d^{10})$ polarises $Cl^{-}$ more strongly than $Na^{+} (2p^{6})$, making $CuCl$ more covalent.
Case Study Based Questions on Fajan’s Rules
Case Study:
Two compounds, $NaCl$ and $CaCl_{2}$, both contain chloride anions. However, $NaCl$ is more ionic, highly soluble in water, and has a higher melting point ($1073 ,K$). On the other hand, $CaCl_{2}$ is less soluble, more covalent, and has a lower melting point ($1045 ,K$).
Q1. Why does $CaCl_{2}$ have greater covalent character than $NaCl$?
Answer: Because $Ca^{2+}$ has higher charge than $Na^{+}$, so it exerts stronger polarising effect on $Cl^{-}$.
Q2. Which Fajan’s Rule applies here?
Answer: Greater charge on cation → stronger polarisation → more covalent character.
Q3. What property of melting points supports the covalent nature of $CaCl_{2}$?
Answer: $CaCl_{2}$ has a lower melting point than $NaCl$, which indicates higher covalent character.


