Anand Classes explains Bond Order, Stability, and Electronic Configuration of Molecules such as H₂, H₂⁺, Li₂, Be₂, C₂, and He₂⁺, along with the reason why He₂ molecule does not exist. Based on the Molecular Orbital Theory (MOT), students will understand how the filling of bonding and antibonding molecular orbitals determines the bond order, stability, and magnetic character of a molecule. This concept is essential for mastering the electronic configuration of diatomic molecules, which is frequently asked in Class 11, Class 12, JEE, and NEET Chemistry exams. The topic includes energy level diagrams, solved examples, MCQs, Q&A, Assertion Reason, and Case Study questions for complete preparation. Click the print button to download study material and notes.
Bonding in Some Diatomic Molecules
Let us discuss bonding in some homonuclear diatomic molecules of the elements of the first and second rows of the periodic table.
What is the bonding in a Hydrogen molecule (H2)?
Each hydrogen atom has one electron in 1s-orbital and, therefore, there are two electrons in hydrogen molecule. Both these electrons are to be accommodated in the lowest energy molecular orbital. According to Pauli’s exclusion principle, these two electrons should have opposite spins.
The molecular orbital energy level diagram for H2 molecule is shown in Figure.
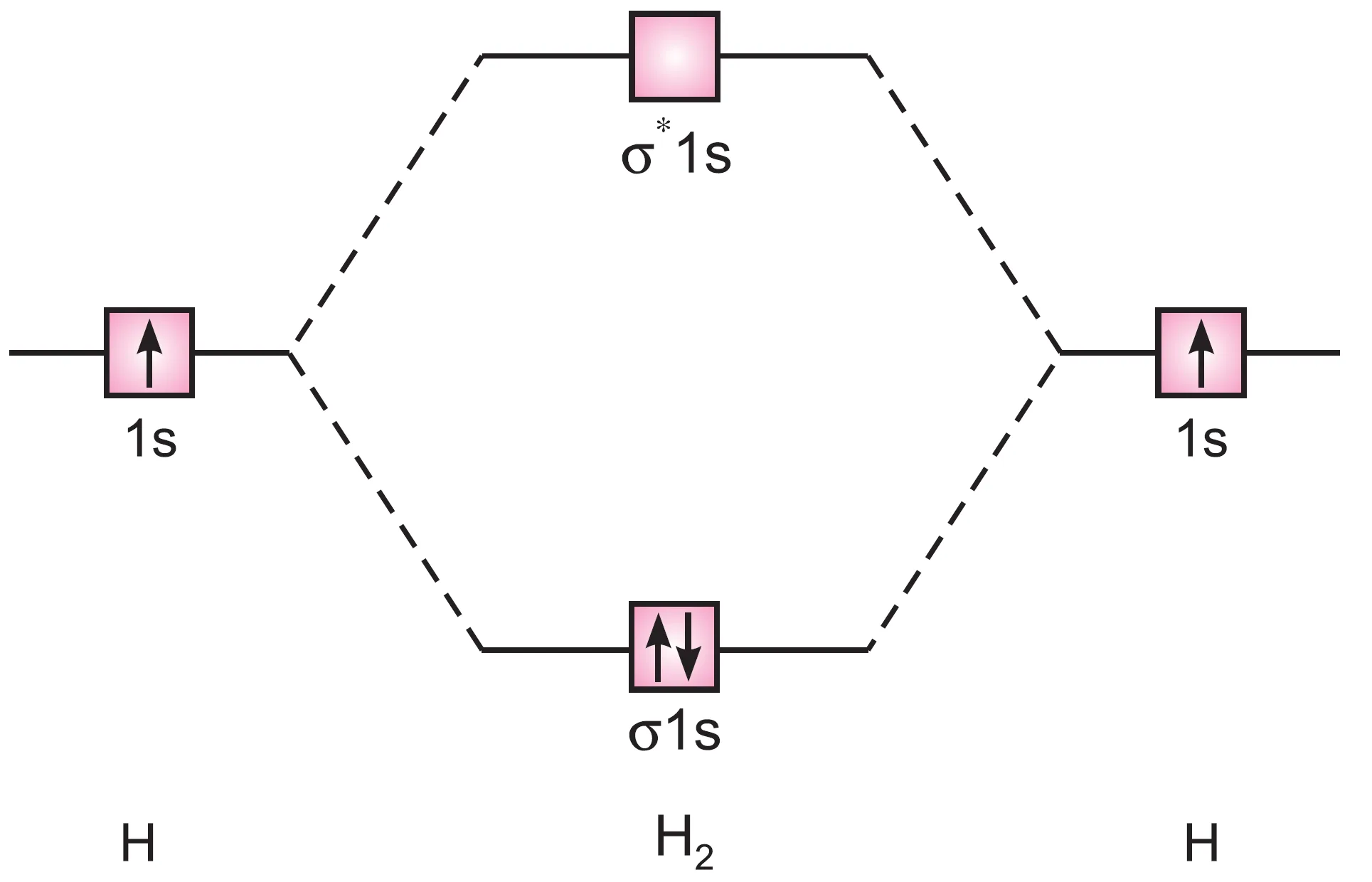
molecule
The molecular orbital electronic configuration of H2 molecule is:
H2 : (σ 1s)2
The bond order in H2 is:
$$ \text{Bond order} = \frac{N_b – N_a}{2} = \frac{2-0}{2} = 1 $$
- Bond dissociation energy = 438 kJ/mol
- Bond length = 74 pm
- Since no unpaired electron is present, H2 is diamagnetic.
What is the bonding in a Hydrogen molecular ion (H2+) ?
This is formed by the combination of a hydrogen atom containing one electron and a hydrogen ion (no electron). Therefore, this ion has only one electron.
The molecular orbital energy level diagram for H2+ ion is shown in Figure.
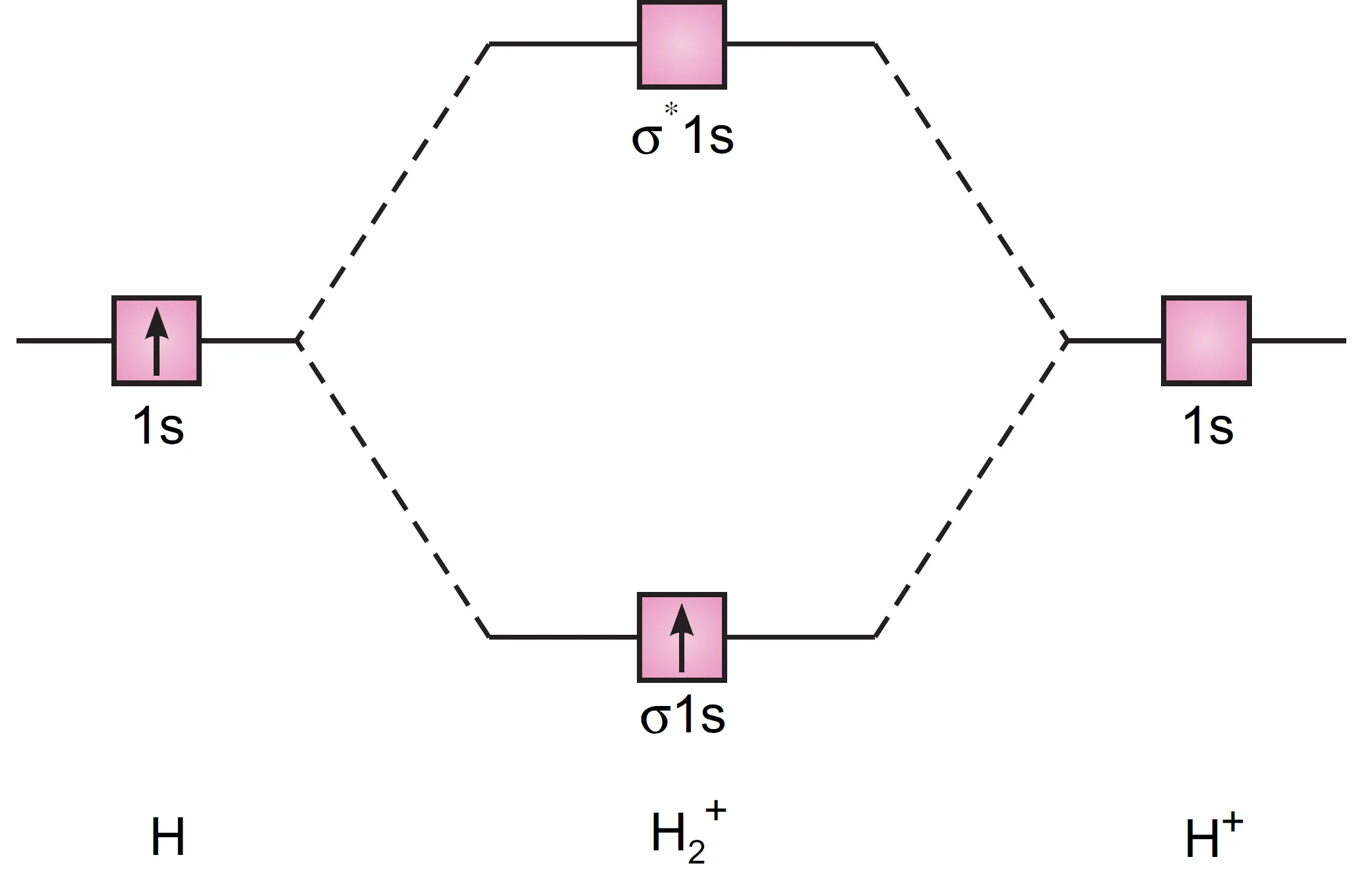
The electronic configuration is:
H2+ : (σ 1s)1
Bond order:
$$ \text{Bond order} = \frac{1-0}{2} = 0.5 $$
- Positive bond order indicates H2+ is stable.
- It has one unpaired electron → paramagnetic.
- Bond length: H2+ = 104 pm, H2 = 74 pm
- Bond dissociation energy: H2+ = 269 kJ/mol, H2 = 458 kJ mol⁻¹
This clearly shows the bond in H2+ is weaker than in H2.
Why does the Helium molecule (He2) not exist?
Each helium atom has two electrons in 1s-orbital, and therefore, there are four electrons in He2 molecule. The electronic configuration for helium molecule may be written as :
He2 : (σ 1s)2 (σ* 1s)2
Molecular orbital energy level diagram for He2 molecule (hypothetical) is shown in Figure.
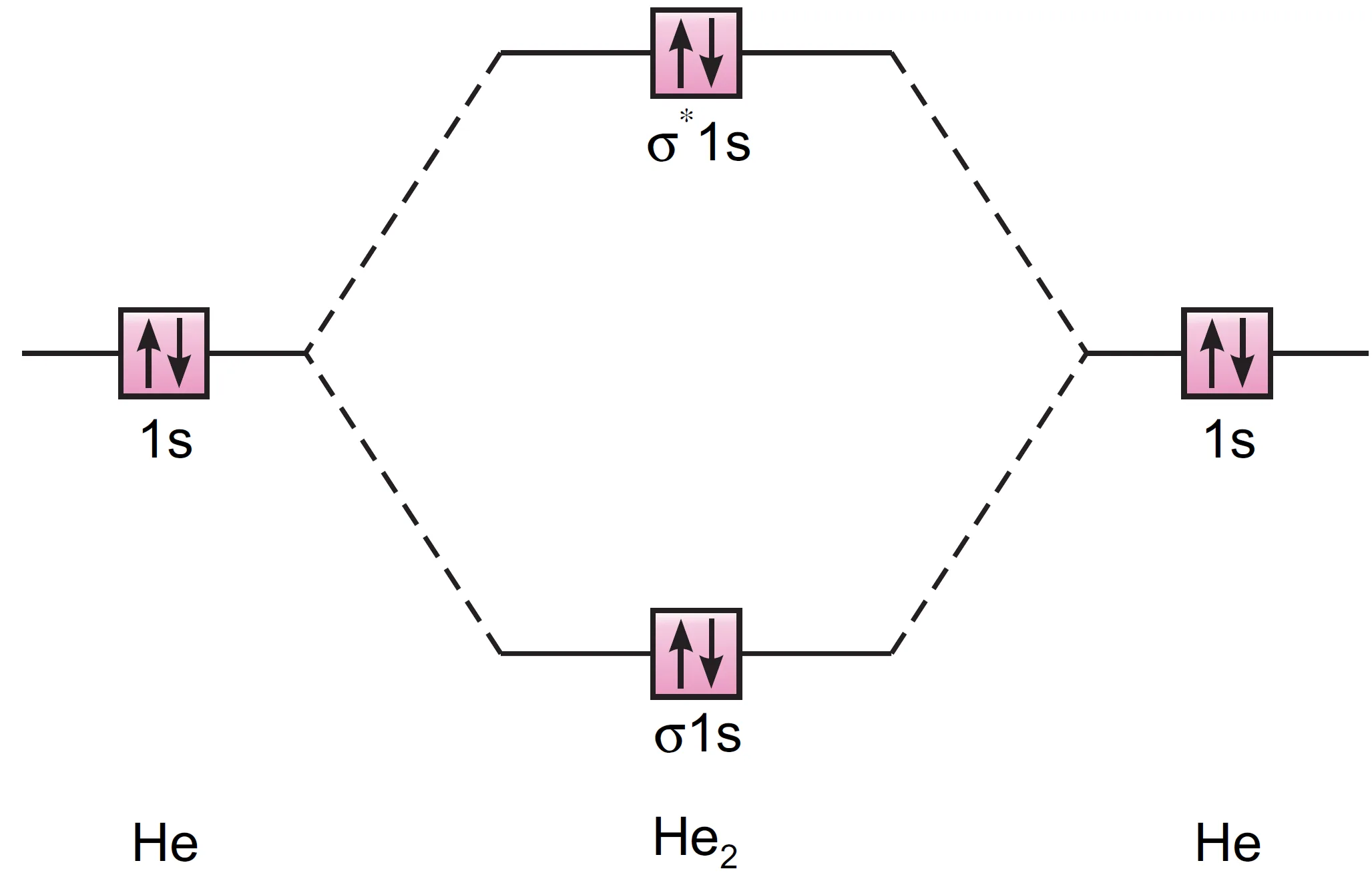
Bond order:
$$ \text{Bond order} = \frac{2-2}{2} = 0 $$
- Zero bond order → no net bonding → He2 does not exist.
- Experimentally, He₂ has never been found.
Why is the Helium molecular ion (He2+) stable?
He2+ contains three electrons (two from He atom and one from He+ ion).
Electronic configuration:
He2+ : (σ 1s)2 (σ* 1s)1
Bond order:
$$ \text{Bond order} = \frac{2-1}{2} = 0.5 $$
- Positive bond order means He2+ is stable.
- Bond dissociation energy = 242 kJ/mol
- It contains an unpaired electron → paramagnetic.
What is the bonding in Lithium molecule (Li2)?
Electronic configuration of Li: 1s2 2s1
Thus, Li2 has six electrons.
Electronic configuration of Li2 :
Li2 : (σ 1s)2 (σ* 1s)2 (σ 2s)2
or simply, KK (σ 2s)2
where KK represents fully filled inner K shells in two atoms i.e. (σ 1s)2 (σ* 1s)2.
From the electronic configuration of Li2 molecule, it is clear that there are 4 electrons in bonding MOs and 2 electrons in antibonding MOs.
Bond order:
$$ \text{Bond order} = \frac{4-2}{2} = 1 $$
- Bond energy = 110 kJ/mol
- Bond length = 265 pm
- All electrons paired → Li2 is diamagnetic
Why Li–Li bond in Li2 is longer and weaker than H–H bond in H2 molecule ?
The comparison of Li—Li and H—H bonds reveals that the sigma bond in Li2 molecule is weaker and much longer than σ-bond in H2 molecule. This may be attributed to the following reasons :
- The 2s orbital of lithium is bigger in size than the 1s orbital. Therefore, the overlapping of 2s—2s atomic orbitals is less effective than the overlapping of 1s—1s orbitals.
- In lithium molecule, the outer electrons in (σ2s) molecular orbital are shielded by the inner 1s electrons. Therefore, the attraction between the nuclei and the electrons in (σ2s) molecular orbital is less.
- Moreover, the inner 1s2 electrons of two lithium atoms cause repulsion between the atoms. Therefore, they do not allow them to come closer.
Hence, Li–Li bond is longer and weaker than H–H bond.
Why does Beryllium molecule (Be2) not exist?
Beryllium (Z = 4) has electronic configuration is 1s2 2s2. In the formation of a diatomic molecule, evidently, two outer electrons of each atom, i.e., four in all, have to be accommodated in molecular orbitals. Two of these go into the bonding (σ2s) orbital while the other two have to go into the anti-bonding (σ*2s) orbital. The molecular orbital electronic configuration is :
Be2 : KK (σ 2s)2 (σ* 2s)2
Bond order:
$$ \text{Bond order} = \frac{2-2}{2} = 0 $$
- Bond order zero → Be2 does not exist.
What is the bonding in Boron molecule (B2)?
The electronic configuration of boron is 1s2 2s2 2p1. The outermost shell of each boron atom contains 3 electrons. When two B atoms combine to form molecule B2, there are 6 electrons which need to be accommodated in the
molecular orbitals of B2. The molecular orbital electronic configuration is :
Electronic configuration:
B2 : KK (σ 2s)2 (σ* 2s)2 (π 2px)1 (π 2py)1
Bond order:
$$ \text{Bond order} = \frac{4-2}{2} = 1 $$
Bond order 1 means the molecule has only one bond. The electrons which contribute towards bonding are the π2px and π2py MOs. This indicates that the molecule is formed by a weak π-bond. The bond dissociation energy of B2 molecule has been found to be 290 kJ/mol and bond length equal to 159 pm.
Since each π2px and π2py MOs contains single electron, the molecule B2 is paramagnetic.
Hence B2 molecule :
- Only one bond (weak π-bond).
- Bond dissociation energy = 290 kJ mol⁻¹
- Bond length = 159 pm
- Two unpaired electrons → B₂ is paramagnetic.
What is the bonding in Carbon molecule (C2)?
The electronic configuration of carbon is 1s2 2s2 2p2. The outermost shell of each carbon atom contains 4 electrons. When two C atoms combine to form molecule C2, there are 8 electrons which need to be accommodated in the
molecular orbitals of C2. The molecular orbital electronic configuration is :
C2 : KK (σ 2s)2 (σ* 2s)2 (π 2px)2 (π 2py)2
Bond order :
$$ \text{Bond order} = \frac{6-2}{2} = 2 $$
Bond order two means Double bond in C2. It may be noted that double bond in C2 molecule consists of both the π bonds because of the presence of 4 electrons in two π MOs. In most of the other molecules, a double bond is made up of a σ bond and a π bond.
The bond dissociation energy of C2 molecule has been found to be 620 kJ/mol and bond length equal to 131 pm.
Further, since C2 molecule does not have any unpaired electron, it is diamagnetic. The diamagnetic C2 molecule has been detected in vapour
phase.
Hence in C2 molecule :
- Bond order two means Double bond in C2 (consists of two π-bonds, not σ + π).
- Bond dissociation energy = 620 kJ/mol
- Bond length = 131 pm
- No unpaired electrons → C2 is diamagnetic.
Short Answer Conceptual Types Questions (SAT)
Q1. Why does $He_{2}$ molecule not exist, while $He_{2}^{+}$ ion exists?
Answer:
- $He_{2}$ has 4 electrons → configuration = $(\sigma_{1s})^{2}(\sigma^{*}_{1s})^{2}$.
- Bond order = $\dfrac{2 – 2}{2} = 0$ → no bond → unstable.
- $He_{2}^{+}$ has 3 electrons → configuration = $(\sigma_{1s})^{2}(\sigma^{*}_{1s})^{1}$.
- Bond order = $\dfrac{2 – 1}{2} = 0.5$ → partial bond → stable.
- Hence, $He_{2}$ does not exist but $He_{2}^{+}$ exists.
Q2. Why is $H_{2}$ molecule diamagnetic but $H_{2}^{+}$ ion paramagnetic?
Answer:
- $H_{2}$: configuration $(\sigma_{1s})^{2}$ → all electrons paired → diamagnetic.
- $H_{2}^{+}$: configuration $(\sigma_{1s})^{1}$ → one unpaired electron → paramagnetic.
Q3. Why is Li–Li bond weaker and longer than H–H bond?
Answer:
- In $Li_{2}$, bond is from 2s–2s overlap, which is less effective than 1s–1s overlap in $H_{2}$.
- Outer $(\sigma_{2s})$ electrons in $Li_{2}$ are shielded by inner $1s^{2}$ electrons.
- Inner $1s^{2}$ electrons cause repulsion, preventing close approach.
→ Result: Li–Li bond energy (110 kJ mol⁻¹) is weaker and bond length (265 pm) is longer compared to H–H (438 kJ mol⁻¹, 74 pm).
Q4. What is the bond order of $B_{2}$ molecule? What does it indicate about its magnetic property?
Answer:
$B_{2}$ has 10 electrons → configuration = B2 : KK (σ 2s)2 (σ* 2s)2 (π 2px)1 (π 2py)1
Bond order = $\dfrac{4 – 2}{2} = 1$.
Since there are two unpaired electrons in $\pi_{2px}$ and $\pi_{2py}$, $B_{2}$ is paramagnetic.
Q5. Why is $C_{2}$ molecule considered to have two $\pi$-bonds instead of one $\sigma$ and one $\pi$ bond?
Answer:
- $C_{2}$ has 12 electrons → configuration = KK (σ 2s)2 (σ* 2s)2 (π 2px)2 (π 2py)2.
- Bond order = $\dfrac{6 – 2}{2} = 2$.
- All bonding electrons are in $\pi$-orbitals, not in $\sigma_{2pz}$.
- Hence, the double bond in $C_{2}$ is made up of two $\pi$-bonds.
Q6. How does bond order affect bond length and stability of a molecule?
Answer:
- Bond order $\uparrow$ → Bond length $\downarrow$ → Stability $\uparrow$.
- Example:
- $H_{2}$ (bond order = 1, length 74 pm) is stable.
- $N_{2}$ (bond order = 3, length 110 pm) is very stable.
- $He_{2}$ (bond order = 0) does not exist.
🎯 Multiple Choice Questions (MCQs)
Q1. The bond order of $H_{2}$ molecule is:
(a) 0.5
(b) 1
(c) 2
(d) 3
Answer: (b) 1
Explanation: Bond order = $\dfrac{2-0}{2} = 1$.
Q2. Which molecule is paramagnetic?
(a) $H_{2}$
(b) $He_{2}^{+}$
(c) $Li_{2}$
(d) $C_{2}$
Answer: (b) $He_{2}^{+}$
Explanation: $He_{2}^{+}$ has configuration $(\sigma_{1s})^{2}(\sigma^{*}_{1s})^{1}$ → 1 unpaired electron → paramagnetic.
Q3. Bond length is smallest in:
(a) $H_{2}$
(b) $Li_{2}$
(c) $B_{2}$
(d) $N_{2}$
Answer: (d) $N_{2}$
Explanation: Bond order of $N_{2} = 3$ → strongest bond → shortest bond length.
Q4. The bond order of $O_{2}$ molecule is:
(a) 1
(b) 2
(c) 3
(d) 2.5
Answer: (b) 2
Explanation: bond order = 2.
Q5. Which of the following does not exist according to MO theory?
(a) $Be_{2}$
(b) $B_{2}$
(c) $Li_{2}$
(d) $O_{2}$
Answer: (a) $Be_{2}$
Explanation: Bond order of $Be_{2} = 0$ → unstable.
🔎 Assertion–Reason Type Questions
Q1. Assertion (A): $H_{2}$ molecule is diamagnetic.
Reason (R): All electrons in $H_{2}$ are paired.
- (a) Both A and R are true, and R is the correct explanation of A.
- (b) Both A and R are true, but R is not the correct explanation of A.
- (c) A is true but R is false.
- (d) A is false but R is true.
Answer: (a) Both A and R are true, and R is the correct explanation of A.
Q2. Assertion (A): $He_{2}$ molecule is not formed.
Reason (R): Bond order of $He_{2}$ is zero.
Answer: (a) Both A and R are true, and R is the correct explanation of A.
Q3. Assertion (A): $B_{2}$ molecule is paramagnetic.
Reason (R): $B_{2}$ has two unpaired electrons in $\pi_{2px}$ and $\pi_{2py}$ orbitals.
Answer: (a) Both A and R are true, and R is the correct explanation of A.
📚 Case Study
Case Study:
The molecular orbital theory explains bonding, stability, bond length, bond energy, and magnetic character of diatomic molecules. For example, $O_{2}$ has a bond order of 2 and contains two unpaired electrons, making it paramagnetic. On the other hand, $N_{2}$ has a bond order of 3, which results in very high bond dissociation energy and short bond length. Molecules like $Be_{2}$ do not exist because their bond order is zero.
Questions:
- What is the bond order of $N_{2}$ molecule?
- Why is $O_{2}$ molecule paramagnetic?
- Which molecule has the strongest bond among $N_{2}$, $O_{2}$, and $F_{2}$?
- Why does $Be_{2}$ molecule not exist?
Answers:
- Bond order of $N_{2} = 3$.
- $O_{2}$ has two unpaired electrons → paramagnetic.
- $N_{2}$ has the strongest bond (bond dissociation energy ≈ 945 kJ mol⁻¹).
- $Be_{2}$ has equal bonding and antibonding electrons → bond order = 0 → unstable.


