Anand Classes provides a complete explanation of sp2 Hybridisation along with the shape and structure of Ethene (C2H4) and Boron Trichloride (BCl3) molecules. Students will learn how one s orbital and two p orbitals mix to form three equivalent sp2 hybrid orbitals, resulting in trigonal planar geometry with 120° bond angles. The topic is explained with diagrams, solved examples, MCQs, Q&A, Assertion-Reason, and Case Study questions, making it ideal for Class 11, Class 12, JEE, and NEET chemistry preparation. Click the print button to download study material and notes.
What is sp2 Hybridisation?
In sp2 hybridisation, one s and two p-orbitals hybridise to form three equivalent sp2 hybrid orbitals. Each sp2 hybrid orbital has one third (33.3%) s–character and two-third (66.7%) p–character. These three sp2 hybrids orbitals remain in the same plane making an angle of 120° with one another. This arrangement is also called trigonal planar arrangement and therefore, the molecules involving sp2 hybridisation of the central atom will adopt trigonal planar geometry.
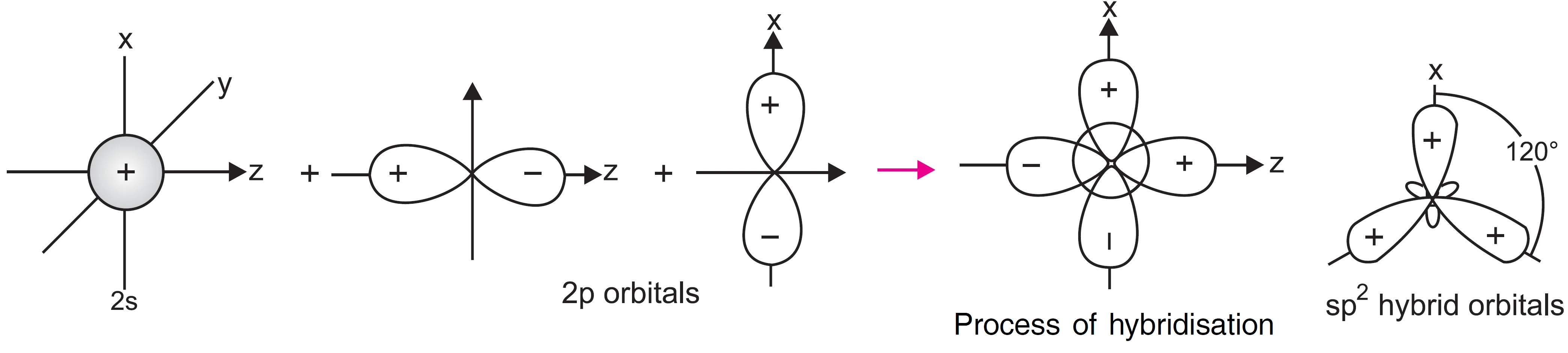
What is the Shape of BCl3 Molecule?
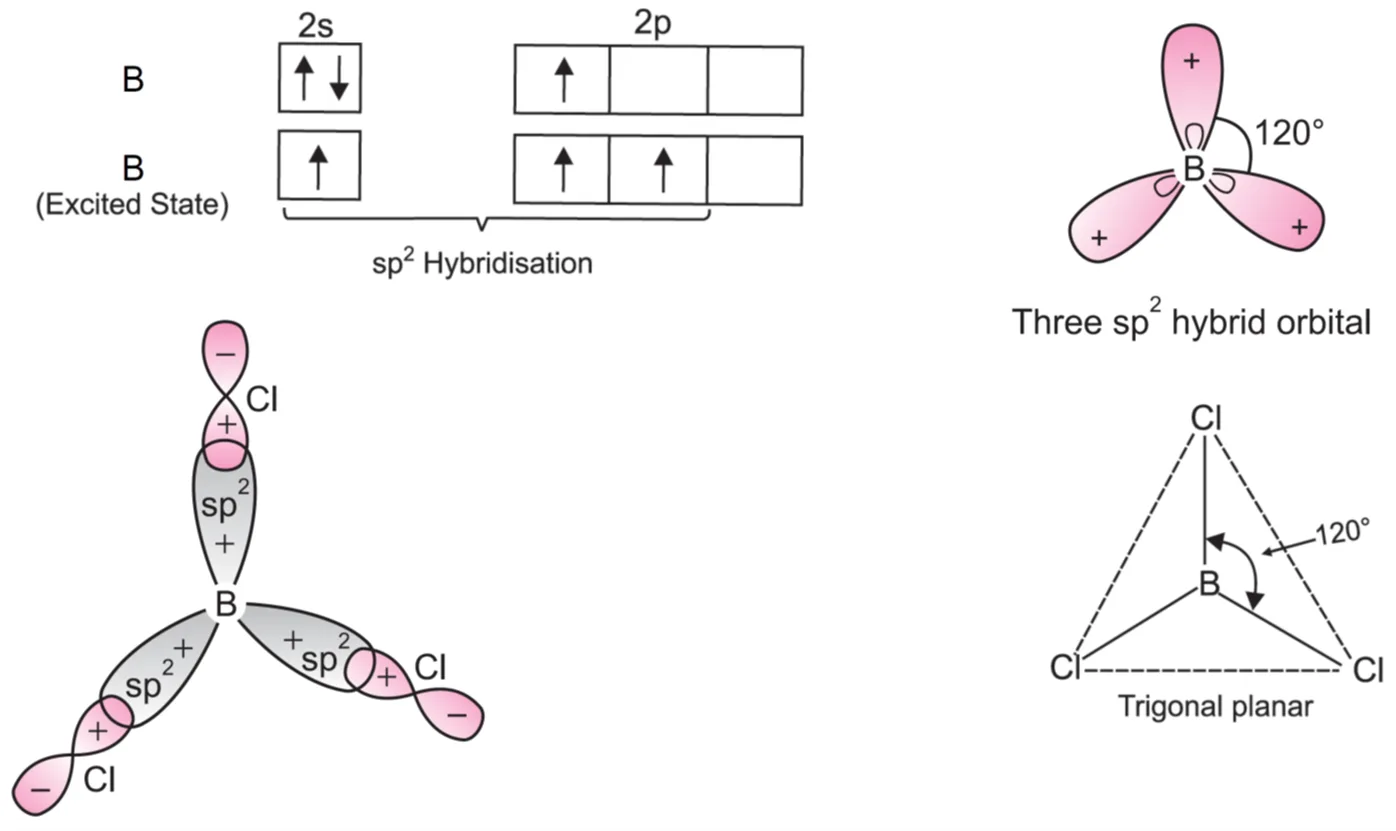
In BCl3, the central boron atom has electronic configuration 1s2 2s2 2p1. To account for trivalency of B, one of the 2s–electrons is promoted to vacant 2p-orbital. Therefore, the excited state of boron has three unpaired electrons.
These three orbitals (one 2s and two 2p) hybridise to form three sp2 hybrid orbitals. These three hybrid orbitals are oriented in a trigonal planar arrangement. Each of these sp² hybrid orbitals overlaps with 2p-orbital of chlorine to form three B—Cl bonds. Thus, BCl3 has trigonal planar geometry and Cl—B—Cl bond angle = 120°.
How Does sp2 Hybridisation Occur in Carbon?
In this type, one s (2s) and two p-orbitals (2px, 2py) present in the valence shell of the excited carbon atom get hybridised to form three equivalent orbitals called sp2 hybrid orbitals.
The orbitals formed are directed towards the three corners of an equilateral triangle with the carbon atom in the centre. The hybridisation is, therefore, known as trigonal hybridisation.
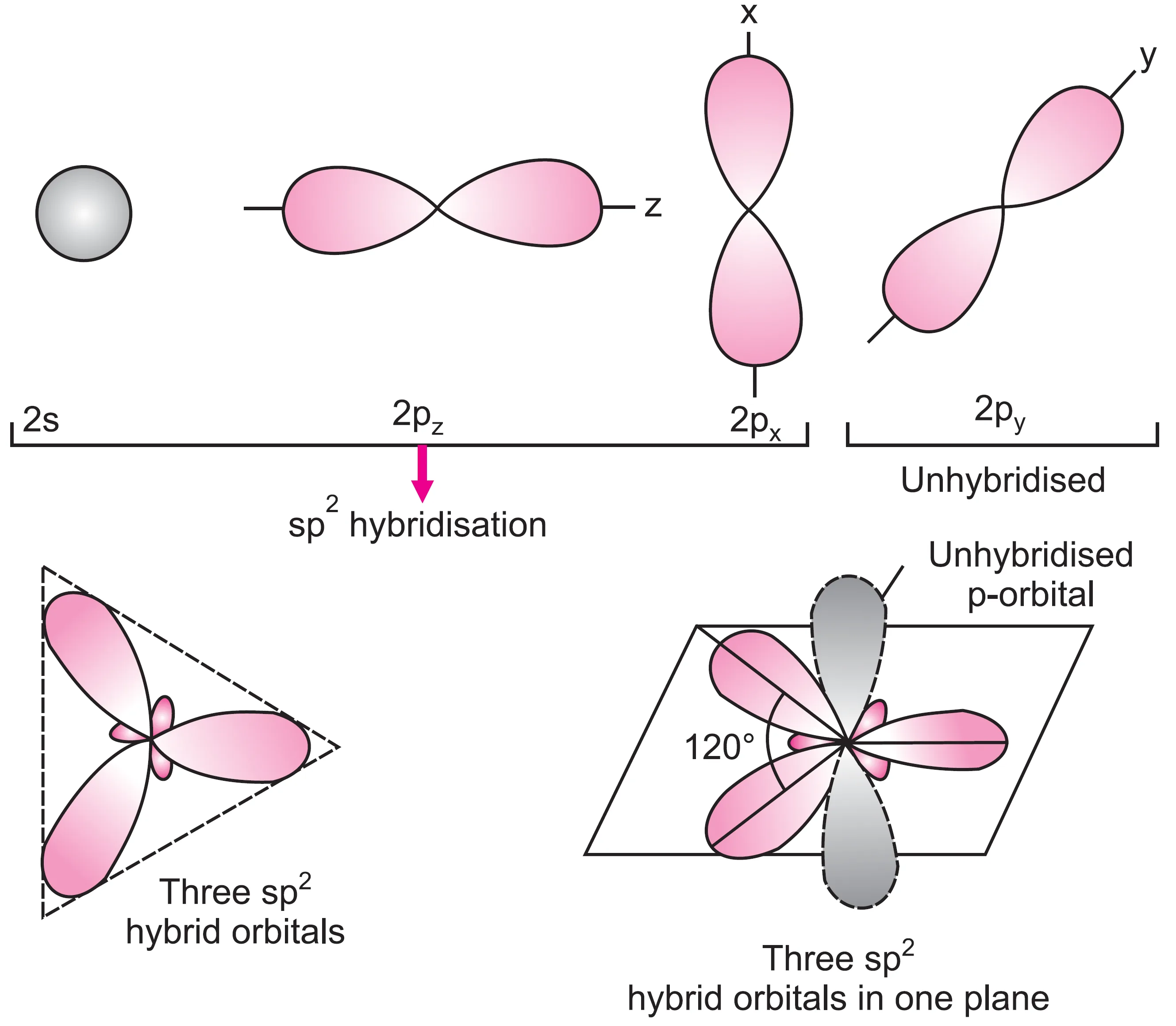
Each sp2 hybrid orbital has one-third s-character and two-third p-character. The bond angle between the two hybridised orbitals is 120°. The unhybridised 2pz orbital of carbon is oriented in a plane at right angles to the plane containing three hybridised orbitals.
How is sp2 Hybridisation Illustrated in Ethene (C2H4)?
Formation of Ethylene or Ethene (H2C = CH2) molecule
Carbon atom has four unpaired electrons in the valence shell in the excited state. In the formation of ethylene, each carbon atom undergoes sp2 hybridisation leaving 2pz orbital unhybridised.
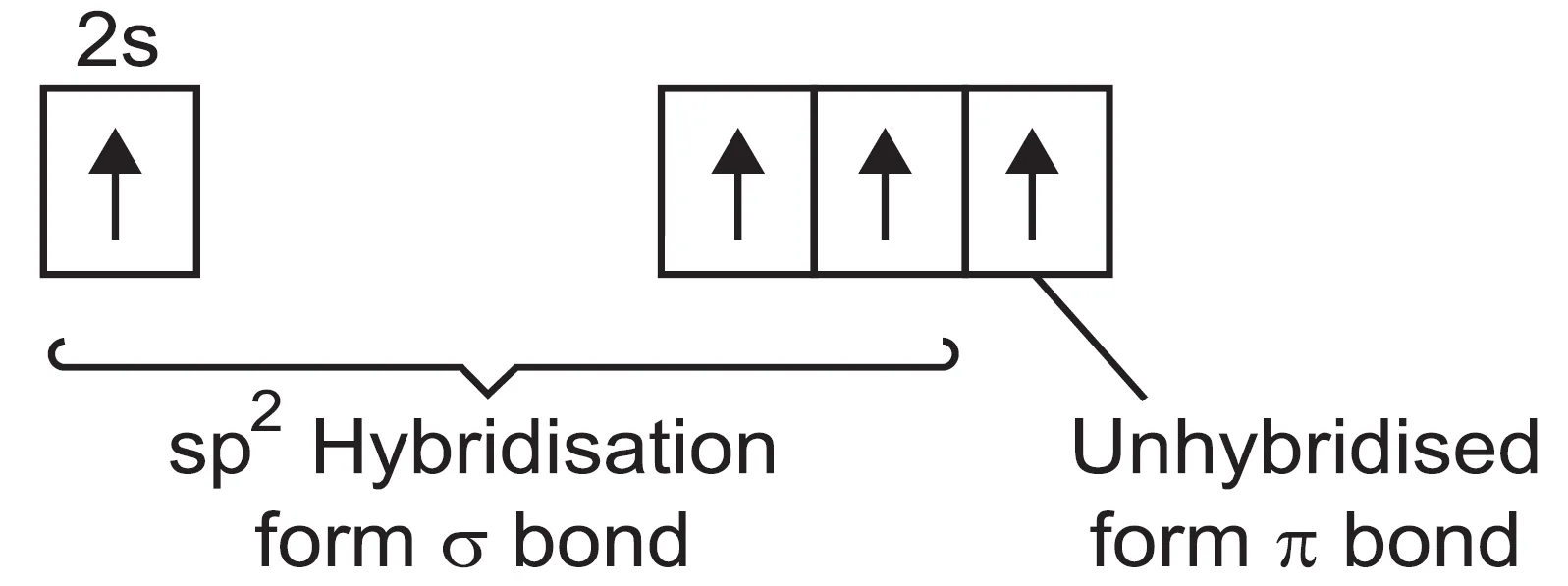
- One sp2 hybridised orbital of one carbon atom overlaps axially with sp2 hybridised orbital of the other carbon atom to form a stable sigma (σ) C—C bond.
- The remaining two sp2 hybrid orbitals of both carbon atoms overlap axially with the half-filled 1s-orbitals of hydrogen atoms forming four C—H sigma (σ) bonds.
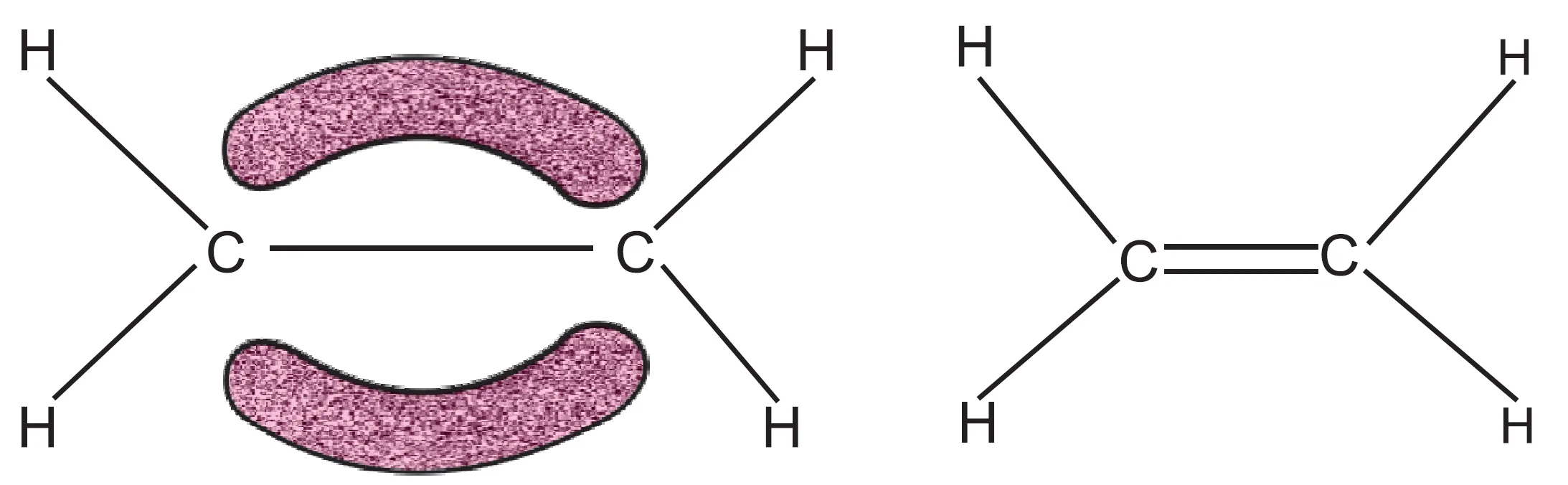
- The unhybridised orbital (2pz) of one carbon atom overlaps sidewise with the similar orbital of the other carbon atom to form weak pi (π) bond.
- The π-bond consists of two equal electron clouds distributed above and below the plane of other atoms.
- Thus, in ethylene, all the six atoms (forming σ-bonds) lie in one plane and π-bond is at a plane perpendicular to the plane of six atoms.
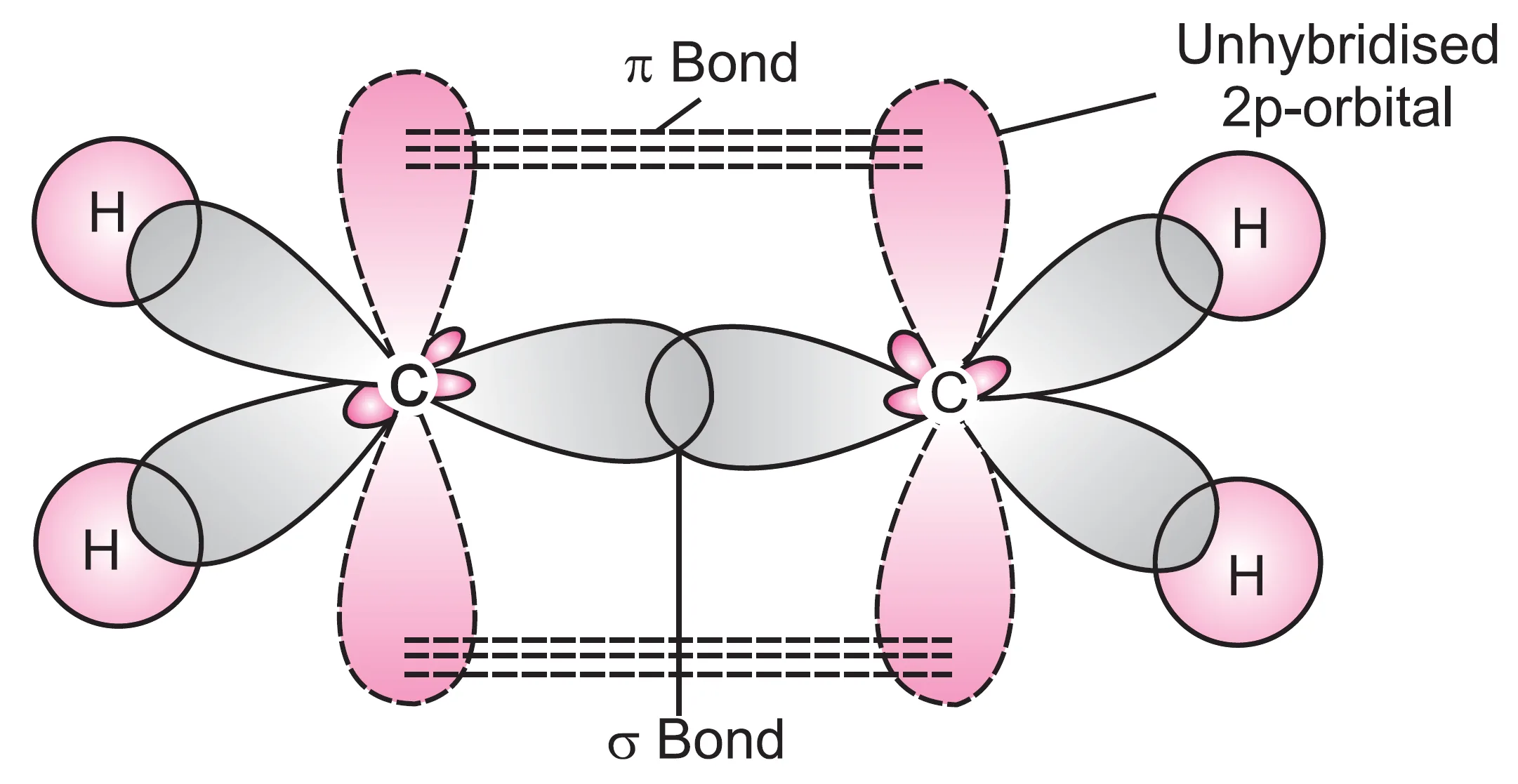
Since there is an equal probability of locating the electron density in both the lobes of the p-orbitals, the same π-bond is formed above and below the internuclear axis representing σ-bond.
As pointed out earlier, π-bond is weaker than the σ-bond since the electrons involved are more diffused in space in the molecular form of a cloud. The presence of such a bond in ethene makes it highly reactive in nature. It is, therefore, regarded as an unsaturated molecule.
What are the Bond Lengths and Bond Angles in Ethene?
In ethene molecule:
- C=C bond length = 134 pm
- C—H bond length = 108 pm
- H—C—H bond angle = 117.6°
- H—C—C bond angle = 121°
Short Answer Conceptual Type Questions (SAT)
Q1. What is sp2 hybridisation?
Answer: In sp2 hybridisation, one s and two p-orbitals hybridise to form three equivalent sp2 hybrid orbitals with 33.3% s-character and 66.7% p-character, arranged at 120° in a trigonal planar geometry.
Q2. What is the shape of BCl3 molecule and why?
Answer: BCl3 has a trigonal planar shape with Cl—B—Cl bond angle = 120° because the boron atom undergoes sp2 hybridisation, forming three equivalent sp2 orbitals that overlap with 2p orbitals of chlorine.
Q3. How does sp2 hybridisation explain the bonding in ethene?
Answer: In ethylene (C2H4), each carbon atom undergoes sp2 hybridisation, forming three sp² orbitals for two C—H σ-bonds and one C—C σ-bond, while the unhybridised 2pz orbitals overlap sidewise to form a π-bond, making the molecule planar.
Q4. Why is π-bond weaker than σ-bond?
Answer: A π-bond is formed by sidewise overlap of unhybridised p-orbitals, where the electron density is more diffused, while a σ-bond is formed by axial overlap, resulting in stronger overlap and higher bond strength.
Q5. Why is ethene considered an unsaturated molecule?
Answer: Ethene is unsaturated because it contains a double bond (one σ and one π bond). The π-bond makes ethene highly reactive as it can easily undergo addition reactions.
Multiple Choice Questions (MCQs) with Answers and Explanation
Q1. In sp2 hybridisation, the orbitals are arranged in:
(a) Linear geometry
(b) Trigonal planar geometry
(c) Tetrahedral geometry
(d) Octahedral geometry
Answer: (b) Trigonal planar geometry
Explanation: In sp2 hybridisation, orbitals arrange in one plane at 120°, giving a trigonal planar arrangement.
Q2. The s-character in sp2 hybrid orbitals is:
(a) 25%
(b) 33.3%
(c) 50%
(d) 75%
Answer: (b) 33.3%
Explanation: sp2 hybrid orbital is formed from 1 s + 2 p orbitals, hence 1/3 = 33.3% s-character.
Q3. Which of the following molecules has sp2 hybridisation at the central atom?
(a) BCl3
(b) CH4
(c) H2O
(d) BeCl2
Answer: (a) BCl3
Explanation: Boron in BCl3 undergoes sp2 hybridisation to form a trigonal planar molecule.
(CH₄ → sp³, H₂O → sp³, BeCl₂ → sp)
Q4. In ethene (C2H4), the type of bonds present are:
(a) 5 σ and 1 π
(b) 6 σ and 1 π
(c) 5 σ and 2 π
(d) 4 σ only
Answer: (a) 5 σ and 1 π
Explanation: Ethene has 4 C—H σ bonds, 1 C—C σ bond, and 1 C—C π bond.
Q5. The C=C bond length in ethene is:
(a) 154 pm
(b) 108 pm
(c) 134 pm
(d) 121 pm
Answer: (c) 134 pm
Explanation: In ethene, C=C bond length = 134 pm, shorter than C—C (154 pm) due to additional π-bond.
Assertion-Reason Type Questions
Q1.
Assertion (A): In BCl3, bond angle is 120°.
Reason (R): Boron atom undergoes sp2 hybridisation.
Answer: Both A and R are true and R is the correct explanation of A.
Q2.
Assertion (A): In ethene, all atoms lie in the same plane.
Reason (R): Each carbon atom is sp2 hybridised, forming trigonal planar geometry.
Answer: Both A and R are true and R is the correct explanation of A.
Q3.
Assertion (A): π-bond is weaker than σ-bond.
Reason (R): π-bond involves sidewise overlap while σ-bond involves axial overlap.
Answer: Both A and R are true and R is the correct explanation of A.
Q4.
Assertion (A): Ethene is highly reactive.
Reason (R): Ethene has a π-bond which is weaker and more reactive than σ-bond.
Answer: Both A and R are true and R is the correct explanation of A.
Case Study Type Question
Case Study:
Ethene (C2H4) is an important hydrocarbon that shows sp2 hybridisation. In ethene, each carbon atom undergoes sp2 hybridisation, leaving one unhybridised p-orbital. The sp² orbitals overlap to form σ-bonds, while the unhybridised p-orbitals overlap sidewise to form a π-bond. This makes the molecule planar, with bond angles close to 120°. Ethene is classified as an unsaturated hydrocarbon due to the presence of a double bond (C=C).
Questions:
- What is the percentage s-character in sp2 orbitals?
- How many σ and π bonds are present in ethene?
- Why is the π-bond weaker than the σ-bond?
- Give the bond length of C=C bond in ethene.
- Why is ethene considered unsaturated?
Answers:
- 33.3% s-character
- 5 σ bonds and 1 π bond
- Because π-bond is formed by sidewise overlap of orbitals, which is weaker than axial overlap in σ-bond.
- 134 pm
- Because it contains a double bond (C=C) making it reactive and capable of addition reactions.


