Anand Classes provides comprehensive study material on Orbital Overlapping (s-s, s-p, p-p), explaining in detail the difference between Sigma (σ) and Pi (π) bonds with clear diagrams, solved examples, MCQs, and Q&A for Class 11 and Class 12 students. This topic is crucial for understanding the Valence Bond Theory, chemical bonding concepts, and their applications in competitive exams like JEE and NEET. Click the print button to download study material and notes.
What is the Orbital Overlap Concept of Covalent Bond?
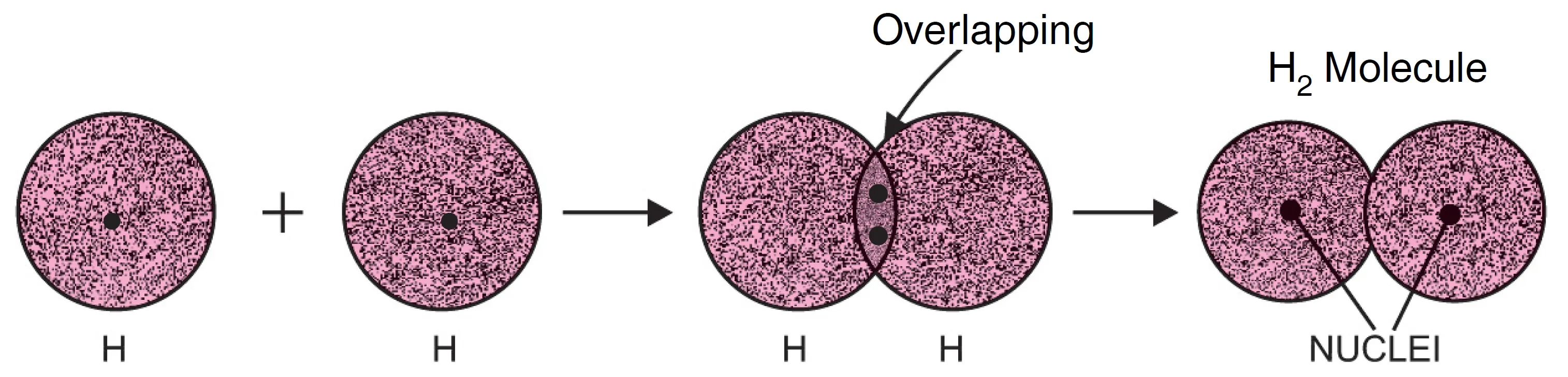
When two atoms approach each other, a partial merger of two bonding orbitals, known as overlapping of the orbitals, occurs. The overlapping of orbitals results in the pairing of electrons.
The strength of a covalent bond depends upon the extent of overlapping:
- The greater the overlapping, the stronger is the bond formed between two atoms.
How Does Orbital Overlap Explain the Formation of a Hydrogen Molecule?
A hydrogen atom has one electron in the 1s orbital. According to Pauli’s exclusion principle, an orbital can hold two electrons of opposite spins.
When two hydrogen atoms, each having one electron with opposite spins, approach each other closely, their orbitals overlap.
Due to this overlapping:
- The two atomic orbitals merge into a bigger cloud called a molecular orbital (see Figure below).
- The molecular orbital contains both electrons.
- These electrons are shared under the above conditions.
Why Does a Covalent Bond Form According to Orbital Theory?
According to orbital theory, the formation of a covalent bond between two atoms results from the pairing of electrons with opposite spins belonging to the valence shells of two atoms.
Thus, in the case of hydrogen (See Figure in above section):
- Two 1s orbitals overlap.
- A stable molecular orbital forms.
- The two hydrogen atoms are held together as a hydrogen molecule (H2).
What are the Types of Overlapping and Nature of Covalent Bonds?
Depending upon the type of overlapping, covalent bonds may be divided into two types:
- Sigma (σ) bond
- Pi (π) bond
What is a Sigma (σ) Bond ?
A sigma bond is formed by the end-to-end (head-on) overlapping of bonding orbitals along the internuclear axis.
- This overlap is known as head-on overlap or axial overlap.
- A sigma bond can be formed by the following types of orbital combinations:
(i) What is s-s Overlapping?
This overlapping occurs between the half-filled s-orbital of one atom and the half-filled s-orbital of another atom along the internuclear axis.
s-s overlapping
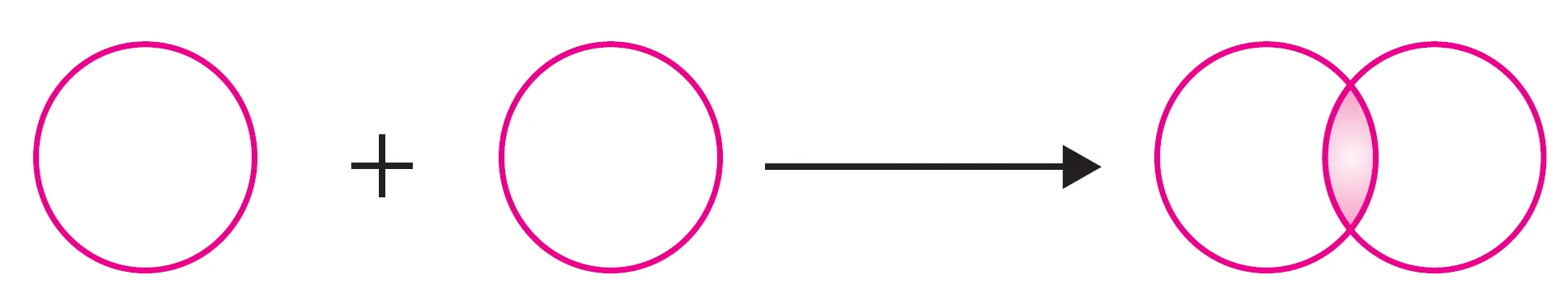
$$ s \; \text{orbital} + s \; \text{orbital} \; \;\longrightarrow \; \; \sigma \; \text{bond}
$$
(ii) What is s-p Overlapping?
This overlapping occurs between the half-filled s-orbital of one atom and the half-filled p-orbital of another atom along the internuclear axis.
s-p overlapping
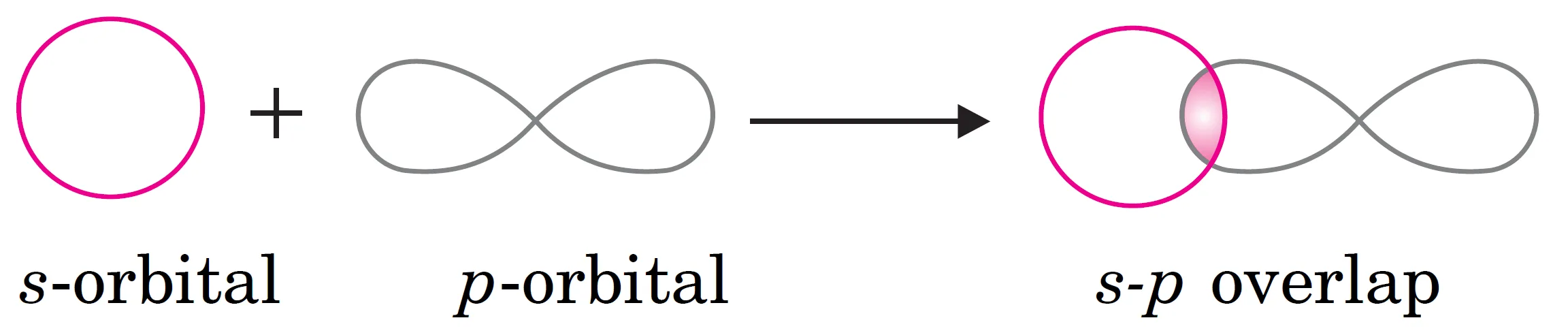
$$
s \; \text{orbital} + p \; \text{orbital} \;\;\longrightarrow\;\; \sigma \; \text{bond}
$$
(iii) What is p-p Overlapping?
This type of overlapping occurs between half-filled p-orbitals of the two approaching atoms along the internuclear axis.
p-p overlapping:
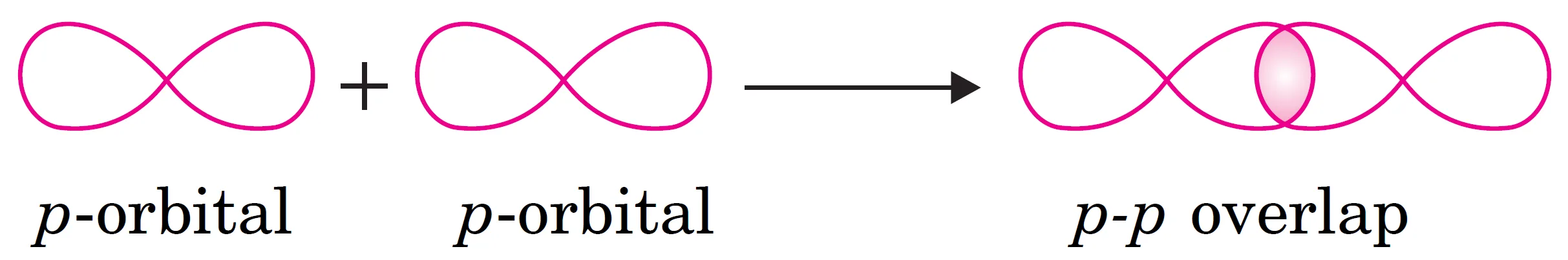
$$
p \; \text{orbital} + p \; \text{orbital} \;\;\longrightarrow\;\; \sigma \; \text{bond}
$$
What is a Pi (π) Bond?
A pi (π )bond is formed by the sidewise overlap of two half-filled p – atomic orbitals of bonding atoms perpendicular to the internuclear axis.
p-p overlapping:
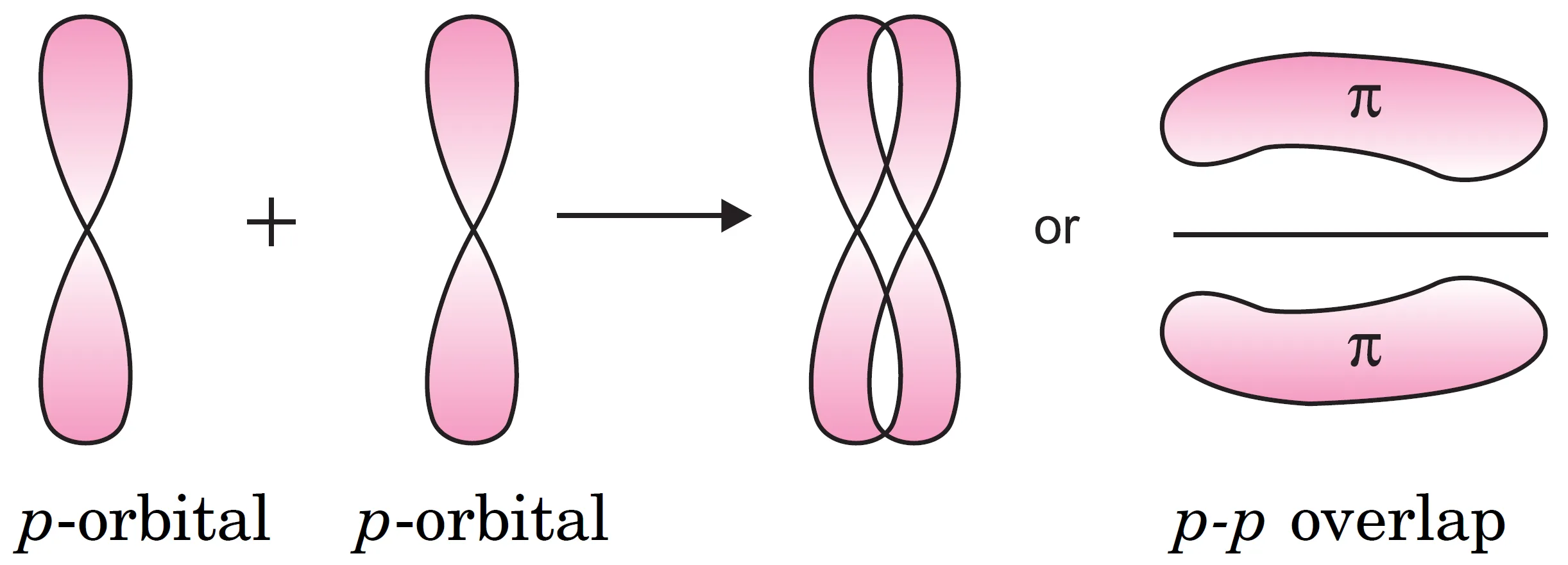
- This overlap is known as sidewise overlap or lateral overlap.
- The atomic orbitals overlap in such a way that their axes remain parallel to each other and perpendicular to the internuclear axis.
- The orbital obtained as a result of sidewise overlap consists of two saucer-shaped charge clouds located above and below the plane of the participating atoms.
What is the Strength of Sigma (σ) and Pi (π) Bonds?
The strength of a covalent bond depends upon the extent of overlapping of the atomic orbitals forming the bond.
- In the formation of a sigma bond (σ-bond), the overlapping of orbitals takes place to a larger extent.
- In the formation of a pi bond (π-bond), the overlapping occurs to a smaller extent.
Therefore,
σ-bond strength > π bond strength
When is a Pi (π) Bond Formed?
A pi (π) bond between two atoms is formed in addition to a sigma bond. Pi (π) bonds are always present in molecules having multiple bonds:
Double bond = σ + π
Triple bond = σ + 2π
Thus, a π-bond never exists alone; it accompanies a σ-bond in double and triple covalent bonds.
Characteristics of Sigma (σ) Bond
- A σ-bond is formed by the end-to-end overlap of filled atomic orbitals along the internuclear axis.
- Overlapping may involve:
- two s-orbitals,
- one s- and one p-orbital, or
- two p-orbitals.
- Overlapping may involve:
- The overlapping can take place to a larger extent, therefore the bond formed is strong.
- The molecular orbital is symmetrical about the internuclear axis.
- There can be free rotation of atoms around the σ-bond.
- A σ-bond may exist alone or along with a σ-bond.
- In the formation of a σ-bond, s-orbitals can participate.
Characteristics of Pi (π) Bond
- A π-bond is formed by the sidewise overlap of two half-filled p-orbitals.
- The overlapping occurs to a lesser extent, therefore the bond is weaker.
- The molecular orbital is discontinuous, consisting of two charge clouds above and below the plane of atoms.
- Due to the overlapping of electron clouds above and below the atomic plane, free rotation of atoms around a π-bond is not possible.
- A π-bond is always present in addition to a σ-bond (never alone).
- s-orbitals cannot participate in the formation of a π-bond.
Difference Between Sigma (σ) Bond and Pi (π) Bond
| Feature | Sigma Bond (σ) | Pi Bond (π) |
|---|---|---|
| 1. Formation | Formed by end-to-end overlap of orbitals along the internuclear axis. | Formed by sidewise overlap of half-filled p-orbitals. |
| 2. Orbitals involved | Overlap may occur between s–s, s–p, or p–p orbitals. | Only p–p orbitals are involved. |
| 3. Extent of overlap | Greater extent of overlap. | Lesser extent of overlap. |
| 4. Bond strength | Strong bond. | Weaker bond. |
| 5. Symmetry | Molecular orbital is symmetrical about the internuclear axis. | Molecular orbital is discontinuous, with charge clouds above and below the plane. |
| 6. Rotation | Atoms can freely rotate around a σ-bond. | Free rotation is not possible around a π-bond. |
| 7. Existence | May exist alone or along with a π-bond. | Always exists with a σ-bond, never alone. |
| 8. Role of s-orbitals | s-orbitals can participate. | s-orbitals cannot participate. |
Short Answer Conceptual Types Questions (SAT)
Q1. Define orbital overlap.
Answer: When two atoms approach each other, their atomic orbitals partially merge. This merging is called orbital overlap.
Q2. Why is a sigma ($\sigma$) bond stronger than a pi ($\pi$) bond?
Answer: In $\sigma$-bonds, orbitals overlap end-to-end, giving greater overlap. In $\pi$-bonds, overlap is sidewise, which is less extensive, hence $\sigma$-bonds are stronger.
Q3. Why can atoms rotate freely around a $\sigma$-bond but not around a $\pi$-bond?
Answer: A $\sigma$-bond is symmetrical along the internuclear axis, allowing free rotation. A $\pi$-bond has electron clouds above and below the plane, which restricts rotation.
Q4. How many $\sigma$ and $\pi$ bonds are present in a triple bond?
Answer: One $\sigma$ bond and two $\pi$ bonds.
Multiple Choice Questions (MCQs)
Q1. A $\sigma$-bond is formed by:
(a) Sidewise overlap of orbitals
(b) End-to-end overlap of orbitals
(c) Overlap of $d$-orbitals only
(d) None of the above
Answer: (b) End-to-end overlap of orbitals
Explanation: A $\sigma$-bond forms when atomic orbitals overlap along the internuclear axis (head-on overlap).
Q2. In which bond can $s$-orbitals participate?
(a) Only in $\pi$-bond
(b) Only in $\sigma$-bond
(c) In both $\sigma$ and $\pi$-bond
(d) Neither $\sigma$ nor $\pi$-bond
Answer: (b) Only in $\sigma$-bond
Explanation: Since $s$-orbitals are spherical, they can only overlap end-to-end, not sidewise.
Q3. Which of the following bonds is the strongest?
(a) Single bond
(b) Double bond
(c) Triple bond
Answer: (c) Triple bond
Explanation:
- Single bond → 1 $\sigma$
- Double bond → 1 $\sigma$ + 1 $\pi$
- Triple bond → 1 $\sigma$ + 2 $\pi$
The presence of multiple overlaps makes triple bond strongest.
Assertion-Reason Type Questions
Q1.
Assertion (A): A $\pi$-bond is always formed in addition to a $\sigma$-bond.
Reason (R): Sidewise overlap cannot take place unless end-to-end overlap already occurs.
Options:
(a) Both A and R are true, and R is the correct explanation of A.
(b) Both A and R are true, but R is not the correct explanation of A.
(c) A is true, R is false.
(d) A is false, R is true.
Answer: (a) Both A and R are true, and R is the correct explanation of A.
Explanation: $\pi$-bonds require orbitals to be already held together by a $\sigma$-bond.
Q2.
Assertion (A): Free rotation is possible around a $\sigma$-bond.
Reason (R): In $\sigma$-bonds, overlapping electron clouds lie above and below the plane of atoms.
Answer: (c) A is true, R is false.
Explanation: Free rotation is possible around $\sigma$-bonds because of axial symmetry, not because of clouds above and below (that is a $\pi$-bond feature).
Case Study Question
Read the passage below and answer the questions:
A covalent bond between two atoms results from the overlap of their atomic orbitals. In hydrogen ($H_2$), each atom has one electron in the $1s$ orbital. When the two atoms come closer, their orbitals overlap, forming a molecular orbital that contains two electrons of opposite spins. Depending on the type of overlap, bonds can be $\sigma$ or $\pi$. In a double bond, one is a $\sigma$-bond and the other is a $\pi$-bond.
Q1. What type of overlap occurs in $H_2$ molecule?
- Answer: End-to-end overlap of $1s$ orbitals → $\sigma$-bond.
Q2. How many $\pi$-bonds are present in a double bond?
- Answer: One $\pi$-bond.
Q3. Which is stronger: $\sigma$-bond or $\pi$-bond? Why?
- Answer: $\sigma$-bond, because the extent of overlap is greater.
Q4. Why can’t a $\pi$-bond exist independently?
- Answer: Because sidewise overlap requires atoms to already be held by a $\sigma$-bond.


