Anand Classes provides detailed Class 11 Chemistry notes on the Shape of PCl₅ (Trigonal Bipyramidal), SF₆ (Octahedral), and IF₇ (Pentagonal Bipyramidal) Molecules explained using VSEPR Theory. Learn how the arrangement of bond pairs around the central atom determines molecular geometry and bond angles, supported with diagrams and examples. These notes also include solved Q&A, conceptual MCQs, and exam-oriented problems for NEET, JEE, and CBSE board exams. Perfect for quick understanding and last-minute revision. Click the print button to download study material and notes.
What is the Shape of PCl5 Molecule? Why is it Trigonal Bipyramidal ?
In PCl5, the central atom, P (Z = 15; 1s2, 2s2 2p6, 3s2 3p3) has five valence electrons. It forms five bond pairs with five Cl-atoms to form a molecule of PCl5. Since there are five electron pairs around the central phosphorus atom, it has trigonal bipyramidal geometry.
In this geometry, all the bond angles are not equal. Three electron pairs are in the same plane at an angle of 120o, while the other two bond pairs are perpendicular to the plane, both making an angle of 90o with the plane. Thus, in this arrangement three bond angles are of 120o each and two are of 90o each.
In this geometry, all five P–Cl bonds are not equal. The three bonds lying in the trigonal plane are called equatorial bonds. Of the remaining two bonds, one lies above and the other below the equatorial plane, both making an angle of 90o with the plane. These bonds are called axial bonds.
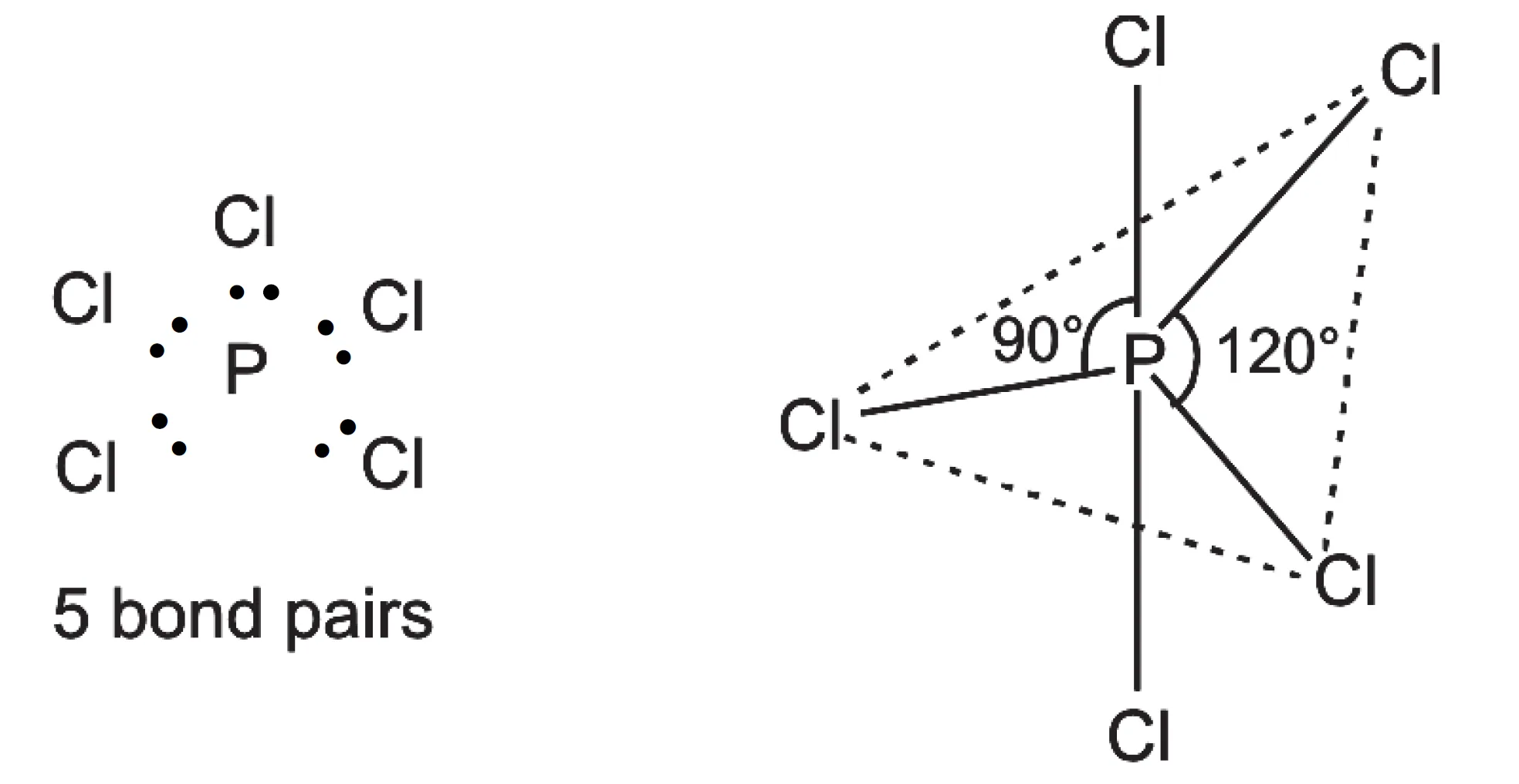
pairs around P-atom
It has been observed that axial bonds are slightly longer than equatorial bonds in this geometry. PF5 has the same shape.
The larger bond length of axial bonds than equatorial bonds :
The larger bond length of axial bonds than equatorial bonds can be explained in terms of the repulsive forces between electron pairs due to different bond angles.
Let us count the repulsive interactions of an axial and an equatorial electron pair:
- The electron pair of an axial bond (say 1) is repelled by three electron pairs (of bonds 2, 3, and 4) at 90o and one (bond 5) at 180o.
- The electron pair of an equatorial bond (say 2) is repelled by two electron pairs (1 and 5) at 90o and two (3 and 4) at 120o.
Since repulsion between electron pairs decreases with increasing angle, the repulsions at 120o and 180o may be neglected compared to those at 90o.
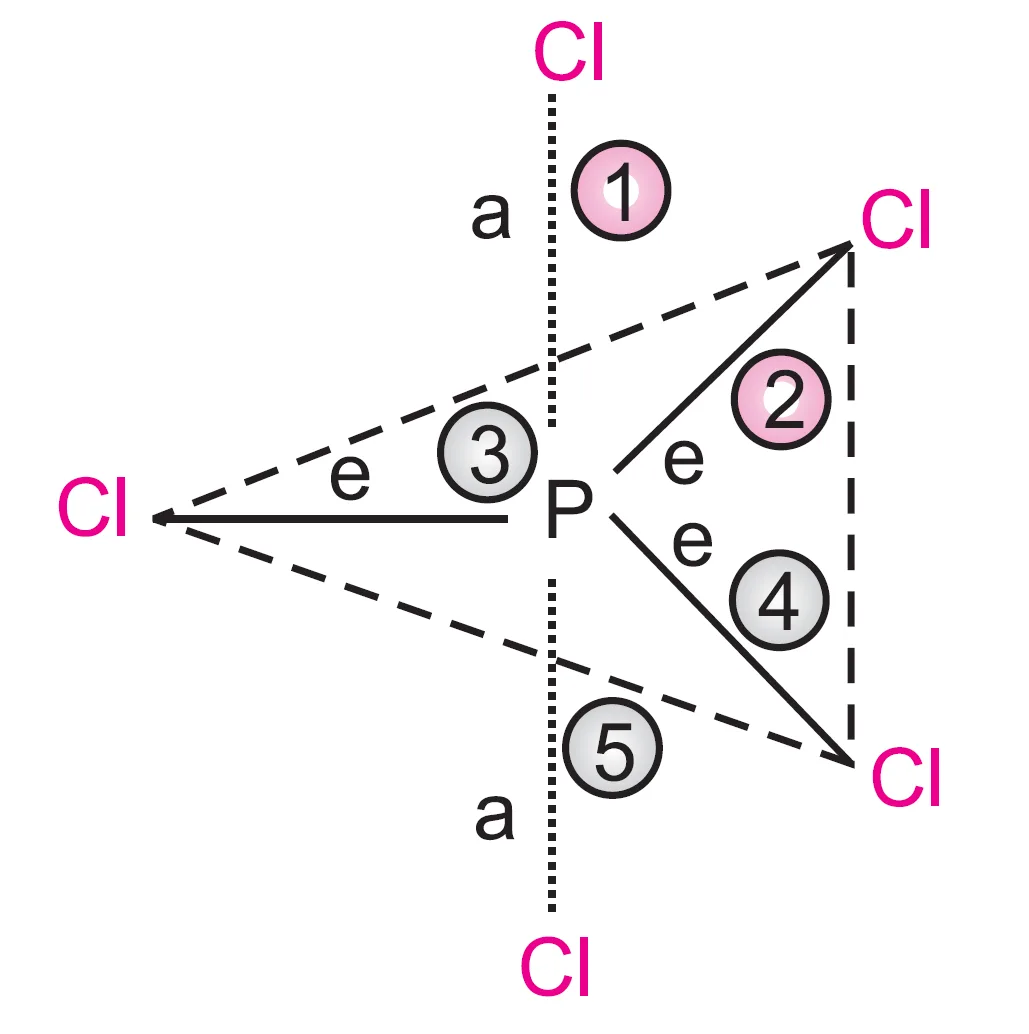
Thus, considering only the 90o repulsions:
- Each axial bond is repelled by three bond pairs.
- Each equatorial bond is repelled by two bond pairs.
Hence, the axial bond pair faces greater repulsion, making the axial bond slightly longer than the equatorial bond.
It may be noted that the structure of PCl5 molecule is unsymmetrical. As a result, it is less stable and is therefore, highly reactive. Therefore PCl5 is dissociate to PCl3.
What is the Shape of SF6 Molecule? Why is it Octahedral?
In SF6, the central S-atom (Z = 15; 1s2, 2s2 2p6, 3s2 3p4) has six valence electrons. Each of these six valence electrons forms a bond with F-atoms, and therefore, the molecule has octahedral geometry.
In this case, all the bond angles are equal and are of 90o each.

around S-atom
TeF6 molecule has the same shape. Other examples of octahedral molecules are SeF6, TeF6, etc.
What is the Shape of IF7 Molecule? Why is it Pentagonal Bipyramidal?
In IF7, the central atom I (Z = 53; … 5s2 5p5) has seven valence electrons. Each of these seven valence electrons forms a bond with F-atoms, and therefore, the molecule has pentagonal bipyramidal geometry.
In this case, all the bond angles are not equal. Five electron pairs are in the same plane at an angle of 72o, while the other two are perpendicular to the plane, both making an angle of 90o with the plane.

pairs around I atom
Thus, in this arrangement:
- Five bond angles are of 72o each (pentagonal plane).
- Two bond angles are of 90o each (axial).
Short Answer Conceptual Type Questions (SAT)
Q1. Why does $PCl_{5}$ have trigonal bipyramidal geometry?
Answer: $PCl_{5}$ has five bond pairs around the central phosphorus atom. According to VSEPR theory, these arrange as three equatorial bonds at $120^{\circ}$ and two axial bonds at $90^{\circ}$ to minimize repulsion, giving a trigonal bipyramidal geometry.
Q2. Why are axial bonds in $PCl_{5}$ longer than equatorial bonds?
Answer: Each axial bond experiences repulsion from three bond pairs at $90^{\circ}$, while each equatorial bond is repelled by only two bond pairs at $90^{\circ}$. Greater repulsion makes axial bonds slightly longer than equatorial bonds.
Q3. Explain the geometry of $SF_{6}$.
Answer: $SF_{6}$ has six bond pairs around sulfur. These arrange at equal $90^{\circ}$ angles in 3D space, giving a regular octahedral geometry.
Q4. What is the bond angle in $IF_{7}$?
Answer: In $IF_{7}$, five F atoms lie in a pentagonal plane ($72^{\circ}$ apart), while two are axial, perpendicular to the plane at $90^{\circ}$.
Q5. Give two examples of molecules with octahedral geometry.
Answer: $SF_{6}, SeF_{6}, TeF_{6}$.
Multiple Choice Questions (MCQs)
Q1. The geometry of $PCl_{5}$ is:
(a) Trigonal planar
(b) Tetrahedral
(c) Trigonal bipyramidal
(d) Octahedral
Answer: (c) Trigonal bipyramidal
Explanation: Five bond pairs arrange as three equatorial and two axial.
Q2. Which of the following molecules is octahedral?
(a) $SF_{6}$
(b) $PCl_{5}$
(c) $IF_{7}$
(d) $BF_{3}$
Answer: (a) $SF_{6}$
Explanation: Six bond pairs give octahedral geometry.
Q3. In $IF_{7}$, the bond angles in the pentagonal plane are:
(a) $90^{\circ}$
(b) $72^{\circ}$
(c) $109.5^{\circ}$
(d) $120^{\circ}$
Answer: (b) $72^{\circ}$
Explanation: Five bonds form a pentagon, each angle $72^{\circ}$.
Q4. Which bonds in $PCl_{5}$ are slightly longer?
(a) Equatorial
(b) Axial
(c) Both are equal
(d) None
Answer: (b) Axial
Explanation: Axial bonds experience greater $90^{\circ}$ repulsion.
Assertion–Reason Type Questions
Q1.
Assertion (A): $PCl_{5}$ has both axial and equatorial bonds.
Reason (R): In trigonal bipyramidal geometry, three bonds lie in a plane and two perpendicular to it.
Answer: Both A and R are true, and R is the correct explanation of A.
Q2.
Assertion (A): All P–Cl bonds in $PCl_{5}$ are equal.
Reason (R): VSEPR theory predicts equal distribution of bond pairs.
Answer: A is false, R is true.
Explanation: Axial bonds are slightly longer than equatorial bonds.
Q3.
Assertion (A): $SF_{6}$ has an octahedral structure.
Reason (R): Six bond pairs arrange themselves at $90^{\circ}$ in 3D space.
Answer: Both A and R are true, and R is the correct explanation of A.
Q4.
Assertion (A): In $IF_{7}$, bond angles in the pentagonal plane are $120^{\circ}$.
Reason (R): A pentagonal bipyramidal geometry requires 120° angles in the plane.
Answer: A is false, R is false.
Explanation: Bond angles in the pentagonal plane are $72^{\circ}$.
Case Study Based Questions
Passage:
According to VSEPR theory, molecules adopt shapes to minimize repulsion between bond pairs. $PCl_{5}$ has trigonal bipyramidal geometry with axial and equatorial bonds of unequal lengths. $SF_{6}$ is octahedral with equal $90^{circ}$ bond angles. $IF_{7}$ adopts a pentagonal bipyramidal structure with five F atoms in a plane ($72^{circ}$ apart) and two axial atoms perpendicular to it.
Questions:
Q1. Which molecule among $PCl_{5}, SF_{6}, IF_{7}$ has unequal bond lengths?
Answer: $PCl_{5}$ (axial bonds longer than equatorial).
Q2. Why is $SF_{6}$ highly symmetrical?
Answer: Because all six bond pairs are equivalent and oriented at equal $90^{\circ}$ angles.
Q3. What is the main difference between $PCl_{5}$ and $SF_{6}$ geometries?
Answer: $PCl_{5}$ → trigonal bipyramidal (5 bonds, unequal bond lengths), $SF_{6}$ → octahedral (6 bonds, all equal).
Q4. State one example of a pentagonal bipyramidal molecule other than $IF_{7}$.
Answer: $IF_{7}$ is the only stable well-known example of pentagonal bipyramidal geometry.


